Translate this page into:
Postpartum Depression: Overview and Management
*Corresponding author: Sarika Chaudhary, Department of Pharmacy, One Beat College of Medical Sciences, Lakhimpur, Uttar Pradesh, India. saruchaudhary37@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chaudhary S. Postpartum Depression: Overview and Management. J Compr Health. doi: 10.25259/JCH_54_2024
Abstract
The mental health of a mother during her pregnancy has a significant impact on the mother, her child, and the family. Psychopathologies such as postpartum depression (PPD) are among the most common. Many women are reluctant to acknowledge their sadness to others after having a child. Postpartum hormone fluctuations may be the cause of the baby blues. PPD is characterized by symptoms of depression, such as ongoing melancholy, disinterest, low self-esteem, disturbed sleep, appetite loss, anxiety, impatience with a hostile attitude toward newborns, self-blame, and feelings of humiliation. There has been a lot published about the symptoms, risk factors, and therapies for PPD, but not much research has been done on why women wait so long to get therapy. Remission, or the resolution of depressive symptoms, is the aim of PPD treatment. It could be required to coordinate with therapists and refer patients to psychiatrists for antidepressant medication. A complete recovery depends on early discovery and treatment.
Keywords
Maternal mental health
Baby blues
Postnatal depression
Risk factor
Sociodemographic factor
INTRODUCTION
Strong emotions can arise with the birth of a child, ranging from joy and excitement to dread and anxiety. However, it can also lead to depression, which is something you might not anticipate. The majority of new mothers encounter the “baby blues” after giving birth, which include mood changes, sobbing fits, anxiety, and trouble falling asleep. The first 2–3 days following delivery are typically when baby blues start, and they can last up to 2 weeks.1-3 Having an infant causes a significant life transition. Although you’re likely expecting to feel happy, you might feel overwhelmed and moody.
Feeling this feeling for a short while is common.4 Your hormone levels decrease after giving birth, which affects how you feel. If you’re experiencing the typical mood fluctuations is called “baby blues” or a more severe illness is called postpartum depression (PPD).
A mood illness known as PPD strikes people within a year of giving birth.5 Low mood and moderate depressive symptoms that are temporary and self-limiting are referred to as postpartum “blues.”6 Parents who are not pregnant can also experience PPD. Companions may experience fatigue and stress as well. Their sleeping and eating schedules may be disturbed. Among the variables that raise the risk are: Being young, having a history of depression, and financial concerns. Surrogate and adoptive parents may also experience these symptoms.7
PPD is characterized by symptoms of depression, such as ongoing melancholy, disinterest, low self-esteem, disturbed sleep, appetite loss, anxiety, impatience with a hostile attitude toward newborns, self-blame, and feelings of humiliation. Changes in eating and sleeping habits, trouble forming a bond with their child, and feelings of worthlessness or hopelessness are among the symptoms that people with PPD may encounter.8 For the patient’s and their child’s health and well-being, PPD must be identified and treated. Untreated PPD can make it difficult to provide for a kid and increase the likelihood that the child will experience long-term developmental problems (such as emotional and behavioral challenges). In addition to complicating family dynamics, PPD raises the chance of suicide.9
PPD may have multiple underlying etiologies, including hormonal fluctuations, genetic predispositions, and psychosocial stressors. The specific cause of PPD is not entirely understood. In those who are vulnerable, the sharp decline in progesterone and estrogen following childbirth, in addition to the stress and lack of sleep that frequently come with taking care of a newborn, can set off depressive episodes.
Gestational diabetes, having boys as babies, having a history of depression, and using epidural anesthesia were identified as risk factors in a meta-analysis of 33 researches. To determine the actual importance of these documented risk factors, particularly the infant’s sex and the use of epidural anesthesia, more research is necessary.8
SOCIODEMOGRAPHIC FACTOR
PPD is thought to affect 17.22% of people worldwide,10 with up to 15% of people having it in the past year in 80 different nations or regions.11 This estimate is higher than the 13% prevalence rate reported in a different meta-analysis of data from high-income countries12 and lower than the 19% prevalence rate of PPD found in studies from low- and middle-income countries. PPD is influenced by several factors, including maternal age (especially among younger women), obstetric stressors, parity, and unplanned pregnancy. Other factors include social factors such as marital status, education level, lack of social support, violence, and financial difficulties.10
The Edinburgh postnatal depression scale (EPDS) is a useful tool for determining the probability that a mother would experience depression symptoms of various intensities. An elevated likelihood of a depressed condition is indicated by a score higher than 13. The overall frequency of PPD in the entire sample was 92 (13.6%), according to the commonly used EPDS, which is used to measure current PPD. According to decreasing order, the largest percentage was found among mothers in Ghana (13 [26.0%] out of 50), followed by Indians (28 [21.7%] out of 129, Egyptians (21 [19.1] out of 110, Yemen (14 [8.5%] out of 164), Iraq (13 [7.7%] out of 169), and Syria (1 [2.3%] out of 43).10
This prevalence was comparable to the 14.3% found in Malaysia by Hairol et al. (2021),13 Malaysian Yusuff et al. (2010) (14.3%),14 and New Delhi’s Nakku et al. (2006) (12.75%).15 In Asia, PPD is a common issue. For instance, in Pakistan, the prevalence of PPD ranged from 28.8% in 2003–36% in 2006– 94% in 2007 and then dropped to 62% in 2021 after a year.16,17 In 2022, however, 45% of Afghans had completed their first labor.18 Primipara accounted PPD in Canada was about 40% in 2015, in India was 22% in 2022, in Malaysia was 22.8% in 2006, again in India was 21.5% in 2019, in Ethiopia’s Tigray zone was 19% in 2017, in Iran ranged from 20.3% to 35% and in China 27.37%. Furthermore, women who were perhaps more at ease talking to family members about their mental health were less likely to suffer from PPD.19-27
PPD RISK FACTORS
Postpartum blues might arise as a result of multiple risk factors. These include a history of significant depression or dysthymia, a history of mood swings linked to the menstrual cycle or pregnancy, a higher number of pregnancies during one’s lifetime, or a family history of PPD.6,28
The following are factors linked to an increased risk of PPD: [Figure 1].29-32
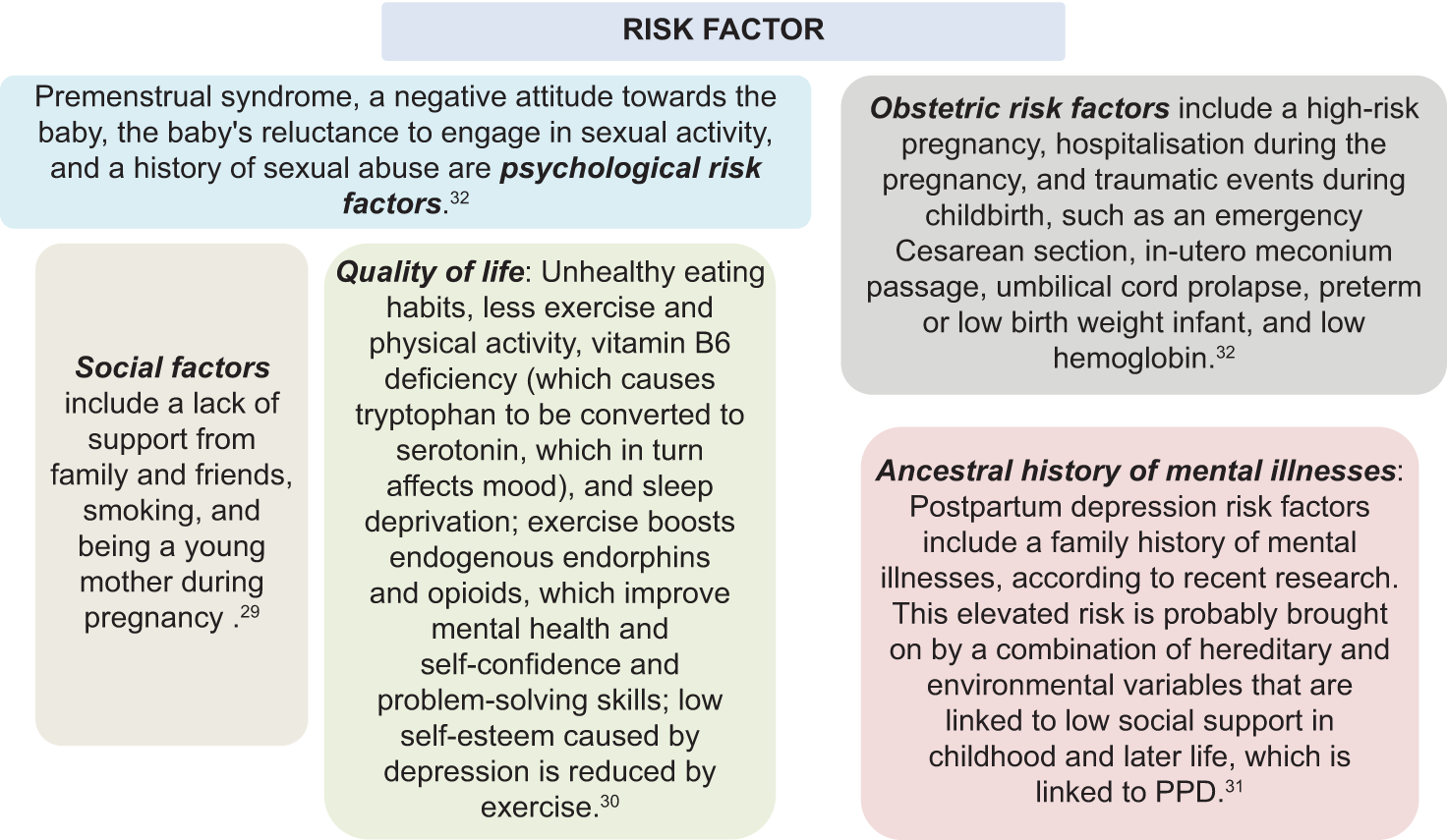
- Risk factor of postpartum depression (PPD).
MANAGEMENT (DIAGNOSIS AND TREATMENT)
When at least five depression symptoms persist for at least 2 weeks, PPD is diagnosed. The majority of experts include symptoms that start-up to a year after giving birth.33 Although the nine symptoms listed below may vary from day to day and be experienced by those who are affected, a diagnosis of PPD must include either anhedonia or depression.
The majority of the day is marked by a depressed mood (either subjective or observed); loss of interest or pleasure (anhedonia); sleep disturbances (insomnia or hypersomnia); psychomotor retardation or agitation; worthlessness or guilt; weariness or lack of energy; suicidal ideation or attempt and recurring thoughts of death; impaired concentration or indecisiveness; and changes in appetite or weight (e.g., a 5% change over 1 month). There may be severe distress and disability as a result of the symptoms. Moreover, neither substance abuse nor a medical issue can be the cause of these symptoms. The incident is neither the result of a mental illness nor has there been a previous manic or hypomanic episode.34
The International Classification of Diseases-10 describes a depressive episode as follows:
The patient experiences a gloomy mood, decreased energy, and activity
A diminished capacity for enjoyment, interest, and focus during typical mild, moderate, or severe depressive episodes
Even with little effort, the patient experiences fatigue, disturbed sleep, and diminished appetite
Commonly felt emotions include guilt, worthlessness, low self-esteem, and low self-confidence
Anhedonia, uncommon early morning awakenings, anxiety, weight loss, decreased appetite, libido, and severe psychomotor impairment are among the somatic signs that are observed.
These symptoms are not sensitive to changing conditions and can fluctuate on a daily basis.
The intensity and quantity of symptoms associated with a depressive episode determine whether it is categorized as mild, moderate, or severe.
PPD shares all of the same symptoms and indicators as nonpuerperal depression, but it also has a history of childbirth. Suicidal thoughts and feelings are among the symptoms, along with low mood, interest loss, altered eating habits, sleep patterns, feelings of worthlessness, and difficulty concentrating. Psychotic symptoms, such as delusions and hallucinations involving voices telling them to kill newborns, can also occur in PPD patients. Poor mother-infant attachments, difficulties nursing, negative parenting styles, marital discord, and inferior results for the child’s physical and psychological development are all possible consequences of PPD. The likelihood of behavioral and mental issues in the progeny decreases when the symptoms subside. The future risk of major depression, bipolar disorder, and PPD is increased in those with a history of PPD episodes. It is also important to consider prior personal and family history of postpartum psychosis and PPD. The likelihood of behavioral and mental issues in the progeny decreases when the symptoms subside. The future risk of major depression, bipolar disorder, and PPD is increased in those with a history of PPD episodes. It is important to take note of any prior PPD and postpartum psychosis diagnoses, both personal and familial.32,34
It is possible to think of peripartum mood disorders as existing on a spectrum, with PPD being more incapacitating and postpartum “blues” being milder and self-limited. Postpartum blues, according to its diagnostic criteria, are temporary and self-limiting. As a result, it resolves itself and does not need any medical intervention other than psychosocial assistance, education, validation, and assurance.35,36 It has been successful in preventing PPD in high-risk patients by combining interpersonal treatment, cognitive behavioral therapy, and counseling. For patients who are at high risk, clinicians ought to recognize and apply these therapies as preventative measures.33
Remission, or the resolution of depressive symptoms, is the aim of PPD treatment. Tracking symptoms should be done with the same screening instrument. A therapy response is defined as a 50% improvement or above. Algorithms can be employed to assist in modifying pharmaceutical dosages when the Patient Health Questionnaire-9, or EPDS, is consistently utilized. Inadequately or mistreated mental health disorders are related to prenatal dangers, as are any pharmacologic medications; the risks of both need to be addressed. To bring the sickness into remission, the lowest effective dosage of medicine should be used. However, it is crucial to prevent undertreatment, which is prevalent in obstetrics. Polypharmacy and switching drugs should be avoided if remission is attainable with the use of a single treatment.33,35 Despite the possibility of improvements being noticed 1 week after oral medication begins, it may take 4–to 8 weeks for evident signs to improve.32,33,35
Treatment for PPD can involve several approaches:
Cognitive counseling: The first-line treatment for patients with mild-to-moderate PPD is psychotherapy. You can get assistance from a psychologist, psychiatrist, or other mental health specialists. You can learn how to make objectives and manage your emotions in therapy. One kind of therapy used to treat PPD is cognitive-behavioral therapy. If you want to create a support network and have a safe space to talk about your feelings, consider joining a discussion group for new parents.
Pharmacological therapy – It includes antidepressant and anti-anxiety pills and a few other medications.
Anti-depressant – For moderate-to-severe depression, a combination of cognitive therapy and antidepressant medicines is advised. An antidepressant may be prescribed by a doctor. You ought to evaluate the benefits and drawbacks of taking medication with your doctor because these drugs can be passed on to your child when you breastfeed. The majority of PPD medications do not pose a significant risk of adverse consequences to your infant. You must be patient as antidepressants take a few weeks to start working. If you feel that the drug is not working, do not stop taking it. To avoid a recurrence of symptoms, medication should be continued for at least 6–12 months once an effective dose is achieved.37 It is not advised to stop taking medicine in the third trimester to reduce the risk of neonatal adaption syndrome because doing so carries a significant chance of recurrence during pregnancy or the postpartum period. Furthermore, unless a steady taper is employed over a period of 2–4 weeks, the sudden withdrawal of both selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors is linked to problems. Gastrointestinal distress, restlessness, anxiety, headaches, weariness, dizziness, insomnia, tremors, myalgias, and electric shocks are some of the symptoms of stopping a medication.
Antidepressants prescribed for PPD include:
SSRIs including sertraline and fluoxetine. SSRIs are the first choice medications for PPD. Consideration should be given to switching to serotonin-norepinephrine reuptake inhibitors or mirtazapine if SSRIs are ineffective. Sertraline or escitalopram are good first-line choices for medical therapy.
Serotonin and norepinephrine reuptake inhibitors, including duloxetine and desvenlafaxine
Norepinephrine-dopamine reuptake inhibitors including bupropion
Tricyclic antidepressants, including amitriptyline or imipramine
Anti-anxiety – These medicines also can pass to your baby through breast milk, so discuss your options with your doctor.
Non-steroidal medications – The Food and Drug Administration (FDA) authorized brexanolone, an injectable neurosteroid that favorably activates Gamma amino butyric acid -A (GABA-A) receptors, in March 2019 with a focus on PPD. When a woman experiences moderate-to-severe depression during her third trimester or after giving birth, benzalone may be prescribed. The Risk Evaluation and Mitigation Strategy Program requires patient enrolment.38 Although brexanolone acts quickly, it can be expensive and difficult to obtain.
There is no evidence to support its effectiveness after 30 days of use or safety during nursing. It is necessary to have inpatient monitoring for enhanced sedative effects, abrupt unconsciousness, and hypoxia during infusion.
Similar to brexanolone, zuranolone is a neuroactive steroid and a GABA-A receptor modulator. On August 4, 2023, the FDA authorized it for the treatment of PPD. For 14 days, an oral dose of 50 mg is administered every night along with a meal that contains 30% fat (700 calories). Zuranolone may be taken alone or in combination with oral antidepressants. It has been proposed that zuranolone may have negative effects on the developing fetus throughout pregnancy and lactation. Zuranolone has few adverse effects and is generally well tolerated.39 Somnolence was the most frequent adverse event that more than 26% of patients experienced.40 Longer than 14 days are not advised when using zuranolone, as safety and efficacy have not yet been investigated.
Non-pharmacological therapy – This therapy may be employed in patients who are not responding to antidepressants and psychotherapy.
A noninvasive technique called transcranial magnetic stimulation employs magnetic waves to activate and stimulate nerve cells in a specific region of the brain.41 Major depressive patients have underactive versions of these cells. For best results, transcranial magnetic stimulation should be performed once daily for 4–6 weeks. Transcranial magnetic stimulation is generally safe and well-tolerated, yet there are a few possible adverse effects, such as headaches, dizziness, pain in the scalp, and twitching of the facial muscles. Seldom occurring severe adverse effects include mania in bipolar illness sufferers, hearing loss if ear protection is insufficient, and seizures.42 Future research is required to fully understand the advantages of transcranial magnetic stimulation for PPD, despite encouraging preliminary results.
It may be suggested that individuals who are not responding to four successive drug trials try electroconvulsive therapy (ECT). Patients with psychotic depression, intent or plans to commit suicide or infanticide, and refusal to eat, resulting in malnourishment and dehydration, benefit from ECT.43,44 Because there are fewer side effects for both the mother and the child, some observational studies have recommended ECT as a safer alternative for patients who are nursing.45,46
Baby blues treatment
If you take care of your body and mind during this difficult period, you should begin to feel better and your baby blues will subside.
Maximize your sleep and take advantage of your baby’s naptime
Eat healthy foods. You will feel better with nutritious fuel in your system
Take a walk. Exercise, fresh air, and sunshine can work miracles
Accept help when offered
Relax. Do not stress about your chores. Just rely on yourself and your baby
Get in touch with other new parents
Neglect alcohol and recreational drugs, as they might worsen mood fluctuations
Consult your healthcare professional for resources if you are struggling with breastfeeding
Try to see or call close friends. Do not isolate yourself.
REPRESSION AND PATIENT EDUCATION
Repression and education are essential components of treating PPD. Proactive teaching regarding PPD should begin in prenatal care, with physicians advising pregnant mothers and their families about the condition’s indications, symptoms, and potential hazards. By raising awareness, new mothers can detect the development of PPD early and seek immediate treatment. Prenatal workshops, relevant leaflets, and talks at regular medical checkups can all be part of an education program. Furthermore, incorporating mental health screenings into postpartum checks can aid in early detection and treatment.32 New moms should have easy access to support systems such as counseling services and support groups, which will provide a safe space for them to express their experiences and get professional help. General and mental health at the population level is the main topics of the National Health Policy (Ministry of Health and Family Welfare, 2017) and the National Mental Health Policy (NMHP) (Ministry of Health and Family Welfare, 2014). By providing accessible, reasonably priced, and high-quality health and social care to everyone throughout their lifespan, within a framework based on rights, NMHP seeks to promote mental health, prevent mental illness, facilitate recovery, encourage destigmatization, and provide the inclusion of those impacted by mental illness. Both the District Mental Health Program and the National Mental Health Program can accomplish this. The provision of care and treatment across all life stages, including pregnancy and childbirth, was the main focus of a national policy plan called Reproductive, Maternal, Newborn, Child, and Adolescent Health + A. Five programs for mothers Pradhan Mantri Surakshit Matritva Abhiyan, Janani Suraksha Yojana, Janani Shishu Suraksha Karyakram, Dakshata, and Laqshya were all related to maternal and newborn health, with a focus on perinatal care and treatment.47 By creating an environment of understanding and support, the stigma associated with PPD can be reduced, enabling more women to seek treatment. Finally, comprehensive repression and instructional methods are critical in lowering the frequency and severity of PPD, resulting in better outcomes for moms and infants.36
CONCLUSION
For both the woman who is suffering from it and her family, PPD may be severely detrimental. Women are more susceptible to PPD due to the interwoven rings created by biological and social variables. This review article indicates that a variety of biological and environmental factors, including lifestyle-related factors, influence serotonin levels in the brain and how well it functions, which, in turn, affects the occurrence or prevention of postpartum depression. Furthermore, a variety of environmental factors, including socioeconomic issues, have an impact on mental health during pregnancy, which can lead to crises and postpartum depression. Screening needs to be a regular part of postpartum care because there are treatments that can effectively stop unnecessary suffering. Antidepressant usage during pregnancy is frequently required for the treatment of depression to prevent maternal psychiatric disease. Commonly prescribed antidepressants used to treat PPD appear to be well tolerated in the postpartum period by nursing mothers and their infants, with minimal rates of side effects. But since the baby will be exposed to the antidepressant through breast milk, the woman and her spouse should talk to their doctor and obstetrician about the advantages and disadvantages of breastfeeding. The focus of postpartum depression prevention programs must therefore be on interpersonal ties between persons to decrease domestic violence, provide social safety, change the lifestyle of women, and improve their capacity to handle crises.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of Interest
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Emotional Anticipation after Delivery-A Longitudinal Neuroimaging Study of the Postpartum Period. Sci Rep. 2017;7:114.
- [CrossRef] [PubMed] [Google Scholar]
- Risk Factors and Course Patterns of Anxiety and Depressive Disorders During Pregnancy and after Delivery: A Prospective-longitudinal Study. J Affect Disord. 2015;175:385-95.
- [CrossRef] [PubMed] [Google Scholar]
- Sociodemographic and Clinical Features of Postpartum Depression among Turkish Women: A Prospective Study. BMC Pregnancy Childbirth. 2015;15:108.
- [CrossRef] [PubMed] [Google Scholar]
- Optimizing the Treatment of Mood Disorders in the Perinatal Period. Dialogues Clin Neurosci. 2015;17:207-18.
- [CrossRef] [PubMed] [Google Scholar]
- Perinatal Major Depression Biomarkers: A Systematic Review. J Affect Disord. 2016;193:391-404.
- [CrossRef] [PubMed] [Google Scholar]
- Non-psychotic Mental Disorders in the Perinatal Period. Lancet. 2014;384:1775-88.
- [CrossRef] [PubMed] [Google Scholar]
- Depression and Anxiety among Postpartum and Adoptive Mothers. Arch Womens Ment Health. 2011;14:335-43.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and Risk Factors of Postpartum Depression in Women: A Systematic Review and Meta-analysis. J Clin Nurs. 2022;31:2665-77.
- [CrossRef] [PubMed] [Google Scholar]
- Improving Maternal Mental Health: Assessing the Extent of Screening and Training about Peripartum Depression. J Matern Fetal Neonat Med. 2023;36:2155042.
- [CrossRef] [PubMed] [Google Scholar]
- Mapping Global Prevalence of Depression among Postpartum Women. Transl Psychiatry. 2021;11:543.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of Patient-reported Outcome Measures for Maternal Postpartum Depression Using the Consensus-Based Standards for the Selection of Health Measurement Instruments Guideline: A Systematic Review. JAMA Netw Open. 2022;5:e2214885.
- [CrossRef] [PubMed] [Google Scholar]
- Rates and Risk of Postpartum Depression-A Meta-analysis. Int Rev Psychiatry. 1996;8:37-54.
- [CrossRef] [Google Scholar]
- Incidence and Predictors of Postpartum Depression among Postpartum Mothers in Kuala Lumpur, Malaysia: A Cross-sectional study. PLoS One. 2021;16:e0259782.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and Risk Factors for Postnatal Depression in Sabah, Malaysia: A Cohort Study. Women Birth. 2015;28:25-9.
- [CrossRef] [PubMed] [Google Scholar]
- Postpartum Major Depression at Six Weeks in Primary Health Care: Prevalence and Associated Factors. Afr Health Sci. 2006;6:207-14.
- [Google Scholar]
- Postpartum Depression: Prevalence and Associated Risk Factors among Women in Sindh, Pakistan. Cureus. 2020;12:e12216.
- [CrossRef] [Google Scholar]
- Postpartum Depression-an Exploratory Mixed Method Study for Developing an Indigenous Tool. BMC Pregnancy Childbirth. 2024;24:49.
- [CrossRef] [PubMed] [Google Scholar]
- Postpartum Depression in India: A Systematic Review and Meta-analysis. Bull World Health Organ. 2017;95:706-17.
- [CrossRef] [PubMed] [Google Scholar]
- Determinants of Postnatal Depression in Sudanese Women at 3 Months Postpartum: A Cross-sectional Study. BMJ Open. 2016;6:e009443.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and Associated Factors of Postpartum Depression among Afghan Women: A Phase-wise Cross-sectional Study in Rezaie Maternal Hospital in Herat Province. Razi Int Med J. 2022;2:63-70.
- [CrossRef] [Google Scholar]
- Postnatal Depression and Socio-cultural Practices among Postnatal Mothers in Kota Bahru, Kelantan, Malaysia. Med J Malaysia. 2006;61:76-83.
- [Google Scholar]
- Prevalence and Predictors of Postpartum Depression among Mothers in the Rural Areas of Udupi Taluk, Karnataka, India: A Cross-sectional Study. Clin Epidemiol Glob Health. 2019;7:342-5.
- [CrossRef] [Google Scholar]
- The Prevalence of Postpartum Depression and Associated Factors: A Hospital-based Descriptive Study. J Clin Anal Med. 2017;8:300-5.
- [Google Scholar]
- Prevalence and Predictors of Postpartum Depression among Pregnant Women Referred to Mother-child Health Care Clinics (MCH) Res J Biol Sci. 2007;2:285-90.
- [Google Scholar]
- Prevalence of Postpartum Depression in Alzahra Hospital in Rasht in 2004. Guilan Univ Med Sci J. 2006;15:97-105.
- [Google Scholar]
- Prevalence and Risk Factors of Postpartum Depression in a Population-based Sample of Women in Tangxia Community, Guangzhou. Asian Pac J Trop Med. 2014;7:244-9.
- [CrossRef] [PubMed] [Google Scholar]
- Exploring Predictors and Prevalence of Postpartum Depression among Mothers: Multinational Study. BMC Public Health. 2024;24:1308.
- [CrossRef] [PubMed] [Google Scholar]
- Risk Factors Associated with the Development of Postpartum Mood Disorders. J Affect Disord. 2005;88:9-18.
- [CrossRef] [PubMed] [Google Scholar]
- Overview of Metabolomic Aspects in Postpartum Depression. Prog Neuropsychopharmacol Biol Psychiatry. 2023;127:110836.
- [CrossRef] [PubMed] [Google Scholar]
- Postpartum Depression Risk Factors: A Narrative Review. J Educ Health Promot. 2017;6:60.
- [CrossRef] [PubMed] [Google Scholar]
- Family History of Psychiatric Disorders as a Risk Factor for Maternal Postpartum Depression: A Systematic Review and Meta-analysis: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2022;79:1004-13.
- [CrossRef] [PubMed] [Google Scholar]
- Postpartum Depression In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2024.
- [Google Scholar]
- Treatment and Management of Mental Health Conditions During Pregnancy and Postpartum: ACOG Clinical Practice Guideline No. 5. Obstet Gynecol. 2023;141:1262-88.
- [CrossRef] [PubMed] [Google Scholar]
- Brexanolone for Postpartum Depression. Am J Health Syst Pharm. 2020;77:336-45.
- [CrossRef] [PubMed] [Google Scholar]
- Postpartum Depression: It Isn't Just the Blues. Am J Nurs. 2006;106:40-50. quiz 50-1
- [CrossRef] [Google Scholar]
- Brexanolone (Zulresso): Finally, An FDA-approved Treatment for Postpartum Depression. Ann Pharmacother. 2020;54:157-63.
- [CrossRef] [PubMed] [Google Scholar]
- Zuranolone, a Neuroactive Drug, Used in the Treatment of Postpartum Depression by Modulation of GABAA Receptors. Pharmacol Biochem Behav. 2024;238:173734.
- [CrossRef] [PubMed] [Google Scholar]
- Zuranolone for the Treatment of Postpartum Depression. Am J Psychiatry. 2023;180:668-75.
- [CrossRef] [PubMed] [Google Scholar]
- Accelerated Transcranial Magnetic Stimulation Provided Rapid Improvement in Depressive, Anxiety, Trauma, and Pain Symptoms in a Woman Experiencing Postpartum Depression. J ECT. 2023;39:e14-5.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of Postnatal Depression with Cognitive Behavioural Therapy, Sertraline and Combination Therapy: A Randomised Controlled Trial. Aust N Z J Psychiatry. 2015;49:236-45.
- [CrossRef] [PubMed] [Google Scholar]
- Postpartum Depression: Pathophysiology, Treatment, and Emerging Therapeutics. Annu Rev Med. 2019;70:183-96.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis, Pathophysiology, and Management of Mood Disorders in Pregnant and Postpartum Women. Obstet Gynecol. 2011;118:708-9.
- [CrossRef] [PubMed] [Google Scholar]
- Biologically Based Treatment Approaches to the Patient with Resistant Perinatal Depression. Arch Womens Ment Health. 2013;16:343-51.
- [CrossRef] [PubMed] [Google Scholar]
- ECT in Pregnancy: A Review of the Literature from 1941 to 2007. Psychosom Med. 2009;71:235-42.
- [CrossRef] [PubMed] [Google Scholar]
- Recommendations for Maternal Mental Health Policy in India. J Public Health Policy. 2023;44:90-101.
- [CrossRef] [PubMed] [Google Scholar]






