Translate this page into:
Meglitinide Drugs Member as a Compendium
*Corresponding author: Feryal Hashim Rada, Department of Clinical Chemistry, College of Pharmacy, Al-Nahrain University, Baghdad, Iraq. firiphd18@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Rada FH. Meglitinide Drugs Member as a Compendium. J Compr Health. 2025;13:12-5. doi: 10.25259/JCH_53_2024
Abstract
Background
Meglitinides (glinides(are a class of antidiabetic drugs used for diminishing the postprandial glucose level by enhancing insulin release from beta-cells of the pancreas.
Objectives
This review is done to insight into the pharmacokinetic and pharmacological aspects of Meglitinides drugs.
Material and Methods
The details of information that present in this review were picked up from numerous scientific sites in online net such as Scopus, PubMed, and others. As well articles with just abstract was omitted.
Results
Glinides involved three types of drugs named Repaglinide (Prandin), Nateglinide (Starlix), and Mitiglinide (Glufast). Glinides can be used alone or co administered with other antidiabetic drug such as metformin, whereas sulphonylurea drug has exception from co administration with glinides members due to the same site of action of both class that lead to excessive side effect.
Conclusion
Glinides are another class of antidiabetic drugs, that act by reducing post-prandial glucose and hemoglobin A1C levels. Weight gain and hypoglycemia are the most common unwanted effects of the use of these drugs.
Keywords
Cytochrome enzyme
Diabetes mellitus
Meglitinides
Repaglinide
INTRODUCTION WITH OBJECTIVES
Diabetes mellitus disorder is the most commonly distributed chronic endocrine disease; it occurs due to impaired insulin release with or without insulin resistance. Thereafter, caused hyperglycemia. Many causes (such as obesity) and diseases can mutually affect diabetes and enhance the release of cytokines associated with it. Diabetes mellitus is categorized depending on the etiology of the disease into many types, the most popular types are type 1 and type 2 diabetes mellitus.1
Many antidiabetic drug classes with different mechanisms are present nowadays that involve sulfonylurea, thiazolidinediones, and anti-dipeptidyl peptidase.2,3 Meglitinides class of drugs are pancreatic beta-cell stimulators that upsurge the release of insulin and are structurally not related to the sulphonylurea drugs. It is used for the management of type 2 diabetes and acts by locking ATP-dependent potassium channels of the beta-cell membrane in the pancreas. Meglitinides only intake shortly before meals to ameliorate post-prandial insulin release. It cannot be used when the meal is canceled or skipped.4,5
Postprandial hyperglycemia provokes endothelial abnormal function, inflammatory reactions, and oxidative stress which lead to increased microvascular and macrovascular complications and hence cardiovascular diseases such as coronary artery disease and stroke. Thereafter, the reduction of postprandial hyperglycemia is important.6 Glinides are characterized by a more rapid onset and shorter duration of action than sulphonylureas. Their site of action is pharmacologically distinct from that of the sulphonylureas.7
Meglitinides class included Repaglinide (Prandin), Nateglinide (Starlix), and Mitiglinide (Glufast). The chemical structure of these drugs is viewed in Figure 1.8 Repaglinide, a carbamoyl methyl benzoic acid derivative, was the first member of the class. It is licensed for use as a monotherapy complementary to diet control, and exercise, or in combination with metformin. Sulfonylureas drugs such as gliclazide and glipizide are a contraindication for co-therapy with meglitinides. Nateglinide was later introduced and is derived from the amino acid d-phenylalanine. It is only permitted for dual therapy with metformin when metformin alone is insufficient. Only the mitiglinide drug has not yet been licensed by the Food and Drug Administration.5,9 This review is executed to insight into the pharmacokinetic and pharmacological aspects of meglitinide drugs.
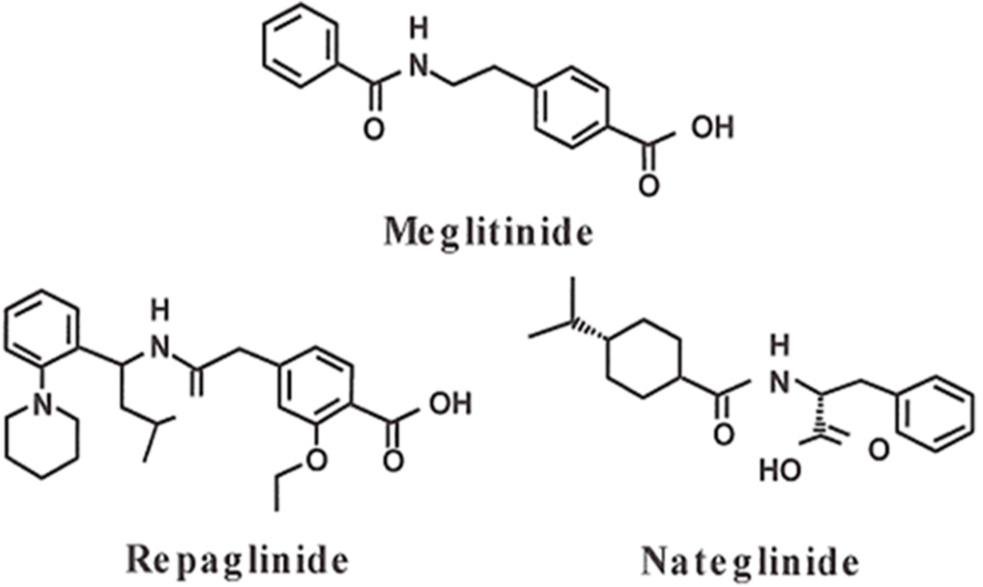
- Structures of meglitinides derivatives.
MECHANISM OF ACTION
Meglitinides (glinides) act by binding to the ATP-dependent potassium channel that presents on the cell membrane of pancreatic beta-cells in a mode analogous to that of sulfonylureas but they have fainter bonded affinity and quicker dissociated from its binding site (sulfonylurea receptor 1). Therefore, simultaneous use of these two therapies was not recommended, as shown in Figure 2.10 Thereafter, the closing of this channel will occur and lead to elevated potassium levels inside the cell and increase the positivity of the membrane, and hence, depolarization occurs that will open the calcium channels voltage-gate and causing inotropic effect on insulin release.11
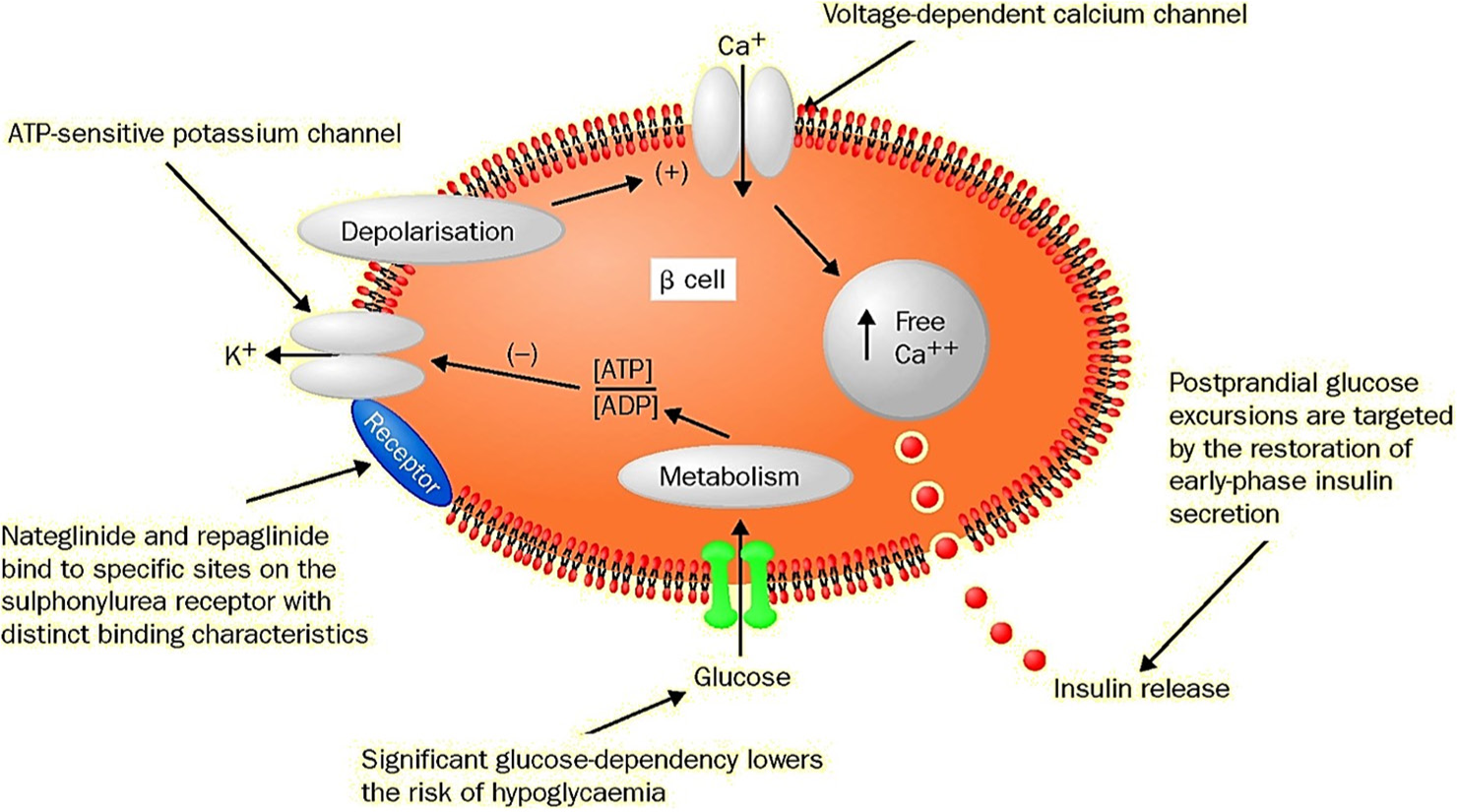
- Mode of action of meglitinides. ATP: Adenosine triphosphate, ADP: Adenosine diphosphate
PHARMACOKINETICS OF MEGLITINIDES
Meglitinides have a quick onset and hasting period of action. Moreover, it must be taken nearly 30 min before the meal. The suggested initial dose for repaglinide is related to the level of hemoglobin A1c, at level <8%, the initial dose is 0.5 mg pre-meal, and at level over 8%, the initial dose is 1–2 mg, whereas dose adjusting is made by measuring fasting glucose level only on 1 week after the starting treatment. Meglitinides are hastily absorbed and have a half-life of around 1–1.5 h and a duration of action of only 4–6 h. The insulin level reverts to its baseline level nearly after 2 h.12,13
Meglitinides are metabolized in the liver by oxidation and conjugation (direct) with glucuronic acid. Likewise, cytochrome enzymes such as CYP2C8 and CYP3A4 are required for their hepatic metabolism. Nateglinide is reported to be metabolized by CYP2C9 enzyme more than CYP3A4. In addition, research reported that repaglinide declines the level of blood glucose post-meal by 5.8 mmol/L and on fasting by 3.1–3.4 mmol/L. As well, repaglinide declines glycated hemoglobin more significantly than sulfonylureas.4,14
ADVERSE EFFECTS OF MEGLITINIDES
Meglitinide drugs cause many adverse effects when used alone or in combination with other drugs. Of these side effects are an increase in body weight, diarrhea, joint pain, sinusitis, and hypoglycemia.
The studies found that the body weight increased with nateglinide treatment more than the repaglinide treatment (1.8 kg: 0.7 kg). Likewise, the elevation in body weight present in patients treated with meglitinides was more than those treated with metformin (1 kg each month).15,16
The incidence of hypoglycemia is lower than that for sulfonylureas. Hypoglycemia if not treated leads to neurological side effect that needs medical care and treatment.17 Many studies stated that the incidence of hypoglycemia occurs more in co-therapy with metformin than monotherapy, while peripheral edema is stated in cases of co-therapy of meglitinides with thiazolidinedione. Moreover, meglitinide drugs may increase the risk of cardiovascular disease at a rate lower than sulfonylureas but more than metformin.12
Also, patients with chronic kidney disease have a high incidence of developing hypoglycemia with or without intake of meglitinide. Thereafter, when prescribing meglitinide drugs for those patients’ carefulness and monitoring of glucose levels should be followed to avoid a risk of hypoglycemia.18
DRUG INTERACTION
Meglitinide drugs are metabolized in the liver by specific types of cytochrome enzymes that involve CYP3A4 and CYP2C8. Thereafter, the level of these drugs in the blood is affected by the presence of another drug that made induction or inhibition for hepatic enzyme and need to adjust the dose of meglitinides. Drugs such as rifampin and phenytoin that made induction of hepatic cytochrome enzyme caused decreased level of blood meglitinides and hence led to a decrease in its hypoglycemic effect. Drugs such as ketoconazole, statin, clarithromycin, and gemfibrozil that made inhibition of hepatic cytochrome enzyme caused increased levels of blood meglitinides and hence led to enhanced hypoglycemic effect.19
The intake of meglitinide drugs is a contraindication in patients with type 1 diabetes and ketosis. Likewise, the treatment with meglitinides is inadvisable in patients with liver disease and in the condition that involves coadministration of clopidogrel or insulin-neutral protamine Hagedorn.20
CONCLUSION
Meglitinides (glinides) are a group of antidiabetic drugs considered as another option of treatment for patients who fail to control their glycemic level with other oral antidiabetic agents. Glinides are used to decrease blood levels of glucose after feeding by stimulating the release of insulin from beta-cells in the pancreas. The action of these drugs is hasty outset and scantly persistent as compared to sulphonylureas. Glinide drug metabolizes into an inert material in the liver by the action of cytochrome enzymes and is removed by bile. As such the intake of other drugs that enhance or reduce cytochrome enzymes may affect their pharmacological effect and need adjusting the doses. Weight accession and hypoglycemia are the most common unfavorable effects of the use of glinides.
Ethical approval
This review was approved by the College of Pharmacy, Al-Nahrain University with approval number 1780, dated 14th October 2024.
Declaration of patient consent
Patient consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Oxidative Stress Assessment in Patients with Diabetic Nephropathy. Eur J Pharm Med Res. 2018;5:44-7.
- [Google Scholar]
- Compendious Review on Adipokines of Corpulence. Res J Pharm Tech. 2020;15:4315-8.
- [CrossRef] [Google Scholar]
- Clinical Assessment of Saxagliptin Therapy in Diabetic Patients with Corpulence. GSC Biol Pharm Sci. 2022;21:109-15.
- [CrossRef] [Google Scholar]
- Repaglinide: Prandial Glucose Regulation in Clinical Practice. Diabetes Obes Metab. 2000;2(Suppl 1):S43-8.
- [CrossRef] [PubMed] [Google Scholar]
- Meglitinide Analogues in the Treatment of Type 2 Diabetes Mellitus. Drugs Aging. 2000;17:411-25.
- [CrossRef] [PubMed] [Google Scholar]
- Importance of Postprandial Glucose Control. South Med J. 2001;94:804-9.
- [CrossRef] [PubMed] [Google Scholar]
- Nateglinide and repaglinide for type 2 diabetes. Drug Ther Bull. 2003;41:52-4.
- [CrossRef] [Google Scholar]
- Single-Dose Pharmacokinetics of Nateglinide in Subjects with Hepatic Cirrhosis. J Clin Pharmacol. 2000;40:634-40.
- [CrossRef] [PubMed] [Google Scholar]
- Recommended International Nonproprietary Names (Rec. INN): List 40" (PDF) In: World Health Organization. 2016. p. :187.
- [Google Scholar]
- Insulinotropic Meglitinide Analogues. Lancet. 2001;358:1709-16.
- [CrossRef] [PubMed] [Google Scholar]
- Meglitinide Analogues: A Review of Clinical Data Focused on Recent Trials. Diabetes Metab. 2006;32:113-20.
- [CrossRef] [PubMed] [Google Scholar]
- Bioequivalence of a Fixed-Dose Repaglinide/Metformin Combination Tablet and Equivalent Doses of Repaglinide and Metformin Tablets. Int J Clin Pharmacol Ther. 2018;56:292-300.
- [CrossRef] [PubMed] [Google Scholar]
- The Effect of Prandial Glucose Regulation With Repaglinide on Treatment Satisfaction, Wellbeing and Health Status in Patients with Pharmacotherapy Naive Type 2 Diabetes: A Placebo-Controlled, Multicenter Study. Qual Life Res. 2003;12:413-25.
- [CrossRef] [PubMed] [Google Scholar]
- Mitiglinide: A Rapid-and Short-Acting NonSulfonylurea Insulinotropic Agent for the Treatment of Type 2 Diabetic Patients. Expert Opin Pharmacother. 2008;9:2691-8.
- [CrossRef] [PubMed] [Google Scholar]
- Repaglinide Versus Nateglinide Monotherapy: A Randomized, Multicenter Study. Diabetes Care. 2004;27:1265-70.
- [CrossRef] [PubMed] [Google Scholar]
- Nateglinide Improves Glycaemic Control When Added to Metformin Monotherapy: Results of a Randomized Trial With Type 2 Diabetes Patients. Diabetes Obes Metab. 2002;4:177-86.
- [CrossRef] [PubMed] [Google Scholar]
- Reduction in Glucose Fluctuations in Elderly Patients with Type 2 Diabetes Using Repaglinide: A Randomized Controlled Trial of Repaglinide vs Sulfonylurea. J Diabetes Investig. 2019;10:367-74.
- [CrossRef] [PubMed] [Google Scholar]
- Meglitinides Increase the Risk of Hypoglycemia in Diabetic Patients with Advanced Chronic Kidney Disease: A Nationwide, Population-Based Study. Oncotarget. 2017;8:78086-95.
- [CrossRef] [PubMed] [Google Scholar]
- Drug-Drug and Food-Drug Pharmacokinetic Interactions with New Insulinotropic Agents Repaglinide and Nateglinide. Clin Pharmacokinet. 2007;46:93-108.
- [CrossRef] [PubMed] [Google Scholar]
- Safety Profile of Repaglinide as Used in General Practice in England: Results of a Prescription-Event Monitoring Study. Acta Diabetol. 2006;43:6-13.
- [CrossRef] [PubMed] [Google Scholar]






