Translate this page into:
Journey of Tuberculosis Control in India: From then till now
CITATION: Zaman FA. Journey of Tuberculosis Control in India: From then till now. J Comp Health. 2021;9(1):05-10.
CORRESPONDING AUTHOR: Dr Forhad Akhtar Zaman MD, FIPHA, ACME, Additional Professor & HOD, Dept. of Community Medicine & Family Medicine, All India Institute of Medical Sciences (AIIMS), Guwahati, Assam. E Mail ID: drforhad@gmail.com
-
Received: ,
Accepted: ,
Abstract
The history of Tuberculosis control in India dates back to 1951 with mass vaccination with BCG and it started as a National Programme in 1962. Radical changes in the form of DOTS were incorporated with the start of Revised National Tuberculosis Control Programme (RNTCP) in 1997. Since then, TB control efforts have witnessed many changes in the form of daily DOTS, Injection free regimen for both drug sensitive & drug resistant TB, moving from fixed Categories of treatment regimen to Individualized treatment regimen based on prior Universal Drug susceptibility testing. Flexibility has been incorporated in the programme to accommodate Private practitioners in the form of various incentives. Introduction of Active case finding strategy has helped in early diagnosis leading to prompt treatments. Engagement of Community and leaders from all sectors and various organizations has helped to reach all communities in this fight against TB. India hopes to End TB by 2025 with rechristening the programme to National TB Elimination Programme (NTEP) and bringing in the much needed changes & flexibilities in the programme.
Keywords
TB Control Program in India
DOTS
Universal DST
End TB
INTRODUCTION
Tuberculosis, is an infectious disease caused by a bacterium known as Mycobacterium tuberculosis[1]. The M. tuberculosis complex (MTBC) includes four other TB-causing mycobacterium: M. bovis, M. africanum, M. canetti, and M. microti.[2] Tuberculosis (TB) is one of the top 10 greatest causes of death worldwide with over 1.4 million deaths in 2019[3] and the leading cause of death due to a single agent. TB is one of the major public health problems in India, with the largest reported epidemics according to WHO.[4] The total number of cases reported for the year 2019 was 2,640,000 with incidence rate of 177 per 100,000 populations.[5] India is ranked at number 38 at a global level in terms of the TB incidence rate. However, being the second most populous country in the world, it has 27% of global burden of TB and has the highest number of TB cases occurring annually. [5] India stands at the 38th position globally, in terms of incidence of Multi drug resistant TB, however it also has the highest number of MDR TB. Nine States account for 65% of the total TB burden - Assam, Bihar, Karnataka, Madhya Pradesh, Maharashtra, Rajasthan, Tamil Nadu, Uttar Pradesh, West Bengal.[5]
History of TB in India
The ancient text Sushruta Samhita described TB in 606 BCE.[6] India established its first TB sanatorium in Tiluania near Ajmer in Rajasthan in 1906[7]. The first TB medical dispensary was established in the year 1917. By 1925 diagnosis of TB via X-Rays became possible. Post independence, the Indian government launched various regional and national programmes for the reduction of TB cases in India. A BCG vaccine production centre was started in Guindy, Madras with the support of WHO and UNICEF after which mass BCG campaign was carried out in the year 1951.[7]
Evolution of TB Control Programmes in India[8,9]
1947: TB Division under the Director General of Health Services
1951: Mass BCG Campaign
1956: Tuberculosis Research Centre (TRC) was established in Chennai (ICMR, WHO, BCMR and Government of Chennai)
1959: National Tuberculosis Institute (NTI) was established at Bangalore
1961: 1st Model District Tuberculosis Centre (DTC), Annantapur district, Andhra Pradesh
1962: National Tuberculosis Programme (NTP)
1961-1986: Era of conventional chemotherapy
1986-1993: Era of short course chemotherapy
1993: Directly Observed Treatment Short Course (DOTS) using intermittent regimen tested
1997: Revised National Tuberculosis Control Programme (RNTCP)
2019: National Tuberculosis Elimination Programme (NTEP)
National Tuberculosis Programme (NTP)
A District Tuberculosis Centre (DTC) was established in Annantapur district of Andhra Pradesh as a pilot project to control the spread of Tuberculosis in 1961[8]. After this, it was launched in the entire country in the form of National Tuberculosis Programme in 1962. NTP primarily focused on the prevention of TB with the use of BCG vaccines along with domiciliary treatment for TB. After a combined review of the programme by the Government of India, WHO and Swedish International Development Agency (SIDA) in 1992, it was concluded that NTP had failed to control the spread of TB, as its diagnosis as well as the treatment rate was just 30%.[8]
Revised National Tuberculosis Control Programme
Post review of NTP, a new revised strategy was launched known as Revised National Tuberculosis Control Programme (RNTCP) first as a pilot project in 1993 and then as a full- fledged National Programme in the year 1997. RNTCP adopted the internationally recommended strategy of Directly Observed Treatment Short Course (DOTS). Smear microscopy was introduced for early diagnosis in a decentralized manner along with strengthening of TB drugs supply.[10] Supervised treatments were carried out in the centre themselves to ensure that the treatment was taken and completed by the patients. RNTCP provided free diagnosis and treatment of the TB patients. The first phase of RNTCP (1998-2005) was to achieve National coverage of DOTS. The second phase of RNTCP was carried out from 2006 -2011. Its objectives were to achieve at least 70% detection of cases as well as to maintain a cure rate of at least 85% of the new smear positive cases. It also strived to accomplish the TB related goals set up in the Millennium Development Goals (MDG) in 2015[11]. Private sector participation was encouraged and provisions were provided for inclusion of DOTS for Drug-resistant TB.
National framework for joint TB/HIV collaborative activities:
In six high HIV/AIDS prevalent states, joint National AIDS Control Program (NACP) and RNTCP started in 2001. It then extended to eight other states by 2007. Some of the salient features of this were[12]
Offer of HIV testing to all TB patients
Intensified TB case finding at ICTCs, ART and Community Care Centres
Linking of HIV-infected TB patients to NACP for HIV care and support (including antiretroviral treatment) and to RNTCP for TB treatment
Provision of Cotrimoxazole Prophylactic Treatment (CPT) for HIV-infected TB patients
Involvement of NGOs/CBOs and affected communities working with NACP and RNTCP for all activities on TB/HIV collaboration.
RNTCP National Strategic Plans [13] NSP Phase I (2012 -2016)
The National Strategic Plan was carried out in two phases. The first phase of NSP was carried out from 2012 and was completed in October 2016. The goal of this plan was "Universal Access to quality TB diagnosis and treatment for all TB patients in the community”. Some of the significant improvements made in this NSP were as follows:
In May 2012 the Government made every TB case registration/notification mandatory even from the private sector.
Complete geographical coverage was achieved in 2013 for the diagnosis and treatment of DR-TB
On 7th June, 2012 Sero-diagnostic tests were banned by the Government in lieu of inaccurate reports.
Set-up of Nikshay which is a web-enabled application launched by the Government of India on 4th June 2012.[14] Its objectives were to create database of all TB cases.
Standards for TB Care in India was set and published in 2014.
End-TB strategy was adopted in 2014.
NTI, Bangalore conducted the first & largest National Drug Resistance Survey during 2016-17.
National Strategic Plan II (2017-2025) [15]
In view of the aim to eliminate TB in India by 2025, Government of India has initiated the National Strategic Plan Phase II (2017-2025) of the RNTCP and the programme was renamed as National Tuberculosis Elimination Programme with effect from 1st January 2020. Four strategic pillars have been incorporated in this plan: Detect/Treat/Prevent/Build (DTPB)
Detect:
Active case finding: screening activity in vulnerable high risk groups (socially backward classes, prison inmates and staffs, TB-HIV, Pregnancy, Diabetes, substance abusers, sexual minorities, malnourished population, etc.)
Notification of all TB cases: Notifications from private care units, labs and chemists were included and failure to take the mandated steps may attract the provisions of Sections 269 the Indian Penal Code (IPC) (Negligent act likely to spread infection of disease dangerous to life) and 270 of IPC. (Malignant act likely to spread infection of disease dangerous to life) The increase in TB notification was also achieved by the expansion of diagnostic facilities at PHCs and scale up of molecular diagnostics (CBNAAT and TruNAAT machines), patient provider support agency (PPSA), availability of free Fixed Drug Combination (FDCs) for the patients in the private sector and schedule H1 implementation in which drugs listed under the Schedule H1 of Drugs and Cosmetic Act, 1940, can be sold only based on the prescription of registered medical practitioners and the pharmacies have to keep records of such prescription for three years for inspection and maintain schedule H1 register. The above activities resulted in a 22% increase in the TB notification from the private sector from 2018.
Early diagnosis and treatment: There was rapid expansion of microscopy centres to improve access. One Peripheral Health Institute (PHI) for every 1 lakh population is a Designated Microscopy Centre (DMC). In tribal, hilly and difficult to reach areas DMC has been established for every 50,000 populations. Currently there are more than 20,110 Microscopy centres, an average of one centre for 75,000 populations. Universal Drug Susceptibility Testing started all over the country under which all TB patients to be tested for Rifampicin Resistance by CBNAAT. Even Rifampicin sensitive cases are to be tested for INH mono resistance. First line & second line LPA is to be done for all Rif resistance cases. In total, 1180 CBNAAT machines have been deployed at district and sub district levels. TrueNat is an indigenous test for detection of TB and resistance to Rifampicin. We are moving towards precision medicine with Individualized Treatment Protocol.
-
Public-private partnership: Incentives provided
Incentive for Treatment supporter - Drug sensitive TB : Rs. 1000/- at completion of treatment & Drug Resistant Case: Rs.5000/- during treatment
Incentive for Informant- An Informant is eligible for incentive of Rs. 500/- for reporting a confirmed TB case & Rs. 500/- on reporting of treatment outcome.
Travel support in Tribal blocks- Rs. 750/- as travel support for all TB patients of tribal blocks
-
Treat
Daily regimen- Daily dose of Directly observed treatment of fixed dose combinations(FDC) drugs according to weight bands was started in 2016 and treatment with injection free regimen was started in 2019. Hence Category 2 regimen was abolished & relegated to history. Nevertheless, there is no restriction on use of Inj. Streptomycin for serious forms of TB e.g. TB Meningitis etc.
Introduction of Cap. Bedaquiline(BDQ) for MDR-TB- Safe, Pain-free, Effective Treatment for Extreme forms of Drug Resistant TB with all Oral Injection Free Regimen was started with the introduction of BDQ.
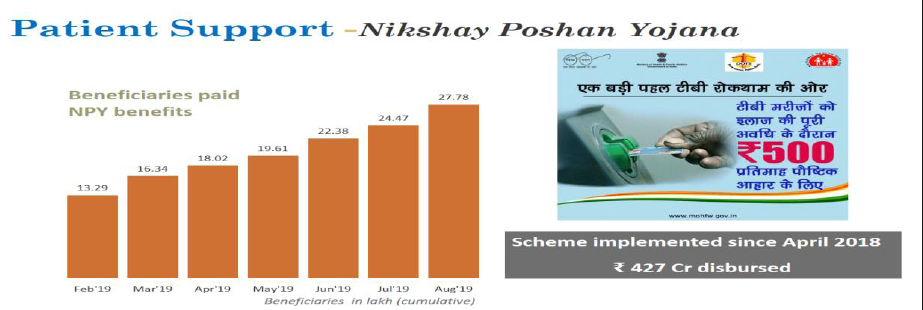
NikshyaPoshan Yojana- Undernutrition is an established risk factor for progression of latent TB infection to active TB and contributes to 7 lakh TB patients annually. To address this critical determinant, NikshayPoshan Yojana (NPY) has been rolled out from April’2018, one of the largest social support schemes for TB patients globally. Under this scheme, each TB patient is eligible for Rs. 500 per month for the duration of TB treatment and benefits are transferred directly in their bank account. Challenges in NPY includes non availability of bank accounts of patients & Patient's non-acceptance of benefits in the private sector where the coverage is a mere 23%.
-
ICT based treatment adherence systems:
Mobile based pill-in hand monitoring tool
SMS reminders, Interactive voice responses
Automated pill loading system
ICT enabled smart cards
Mobile toolkit for patients for treatment compliances
-
Prevent:
Air-borne infection control:
Contact tracing of the diagnosed cases
-
Isoniazid Preventive Therapy (IPT):
For close contact children<6 yrs
For HIV infected children with no active TB but with contact to a positive case or a positive Tuberculin skin test
All children with positive TST on Immunosuppressive therapy
Children born to mothers with TB during pregnancy (treatment for 6 months)
Social determinants of TB to be addressed: poverty, malnutrition, inadequate ventilation, etc.
-
Build:
Translate high level political commitment to action through supportive policy and institutional structures
Restructure NTEP management structure and implementation arrangement
Scale up Technical Assistance at national and state levels
Align and harmonize partners’ activities with programme needs to prevent duplication
Latest initiatives under NTEP
National TB Prevalence survey-
Govt.of India rolled out in October 2019 the World's largest National level TB Prevalence survey, which will not only measure TB prevalence at the national level but also the TB prevalence of 20 states and state/groups. This survey is being implemented by ICMR and its institutes with the support of Central TB Division and the World Health Organization. 5 lakh adults across the country in 625 clusters will be screened with TB symptoms using digital chest x-ray and CBNAAT in specially fabricated vans. Survey results will also help us to understand the health seeking behavior of patients and indirectly that of practitioners, so that more facilitative and enabling environment can be created to accelerate progress towards Ending TB.
Paradigm shift in management of Drug Resistance TB
Shorter MDR TB regimen- All MDR RR TB patient without resistant to Second Line Drugs (SLD)
Bedaquiline (BDQ) containing regimen- MDR RR TB patient resistant to SLD
Delamanid (Dlm) Containing regimen- 7 states for >6 yrs while nationwide access for 6 to 18 yrs
All oral longer regimen- MDR TB patient not eligible for shorter MDR TB regimen.
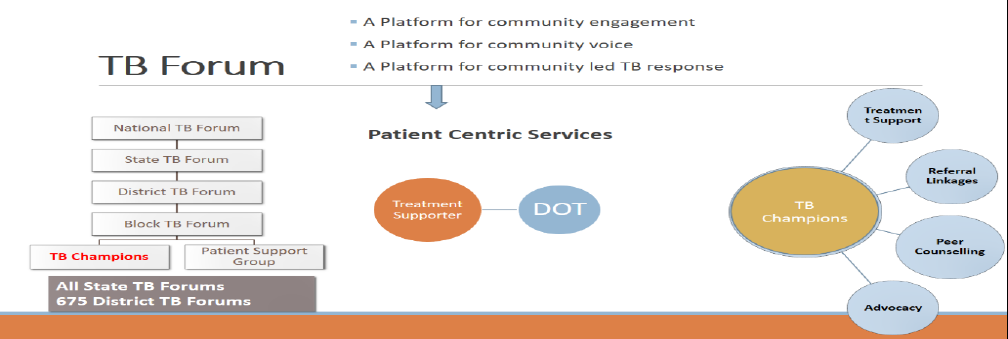
Creation of TB Forums at various levels
TB Forums at National, State, District & Block levels provide an institutional platform to include community as an important stakeholder under the programme to improve the quality of TB services and making them patient-centric. The forums have representation from people affected by TB, elected representatives, policy makers, civil society organizations and programme managers. The TB Forums have the mandate to advice the programme on strategies for engaging communities and increasing community participation in TB Programme, periodically review progress of NGO related activities and involvement of communities and facilitate community financing to sustain TB patient support services through community.
Collaborative Measures
Indian Medical Association has been involved for large scale advocacy on engagement of private health care providers across the country. Continuous Medical Education (CME), Digital Video Modules and Public Relations (PR) strategic communication campaign targeting private health care providers are the key activities being undertaken under the project. In collaboration with Indian Academy of Paediatricians (IAP), the RNTCP has prepared a comprehensive curriculum to train paediatricians on standard protocols for diagnosis and treatment of TB in children. Paediatricians from both public and private sector have been trained through these interactive training modules.
Patient Provider Interface Agency (PPIA) approach
India has successfully demonstrated patient provider interface agency (PPIA) approach for engagement of private health care providers efficiently and reach to patients seeking care from them. The interface agency approach has been transitioned to patient provider support agency (PPSA) and expanded through Joint Effort for Elimination of TB (JEET) project to 48 larger urban cities and 385 districts with support of Global Fund for AIDS, TB and Malaria (GFATM).
“TB HaregaDeshJeetega” advocacy campaign
Under this campaign, Intensive Awareness activities have been arranged in the form of specific information modules for various targeted groups like Self-Help groups/ Panchayati Raj Institutions/ Urban Local Bodies/ Youth groups. Also efforts have been made for Dissemination through Radio/ Social Media / Print/ TV
Latent TB Infection Management
India has the highest burden of Latent TB Infection (LTBI) globally, with nearly 35-40 crores Indian population having latent tuberculosis infection (LTBI), of which 25-30 lakhs develop TB disease annually. At present, programme is providing preventive therapy to all People Living with HIV and child contacts <6 years of TB patients. Scaling up TB preventive therapy and Rolling out newer diagnostic tests and shorter treatment would be key to hasten the decline in TB incidence and key for achieving the target of ending TB by 2025.
CONCLUSION
India has come a long way in curbing TB but still has the highest burden of TB and MDR-TB in the world. Through the programmes and strategies described above, the efforts of the Government has not been in vain as post RNTCP commencement, 90 million people have been tested and 19 million detected and treated. Financial incentives, public-private partnerships, set up of different organizations and committees, extensive network of treatment suppliers, varied ICT modalities, pharmacovigilance, co-morbidities therapy, health education and systemic and standardized reporting and surveillance system are the varied ways how the information of TB, treatment of TB, the after effects of therapy and the high risk groups along with the co-morbidities are being spread around the world. They have played a huge role in bringing India to where it stands today.
Challenges still remains in reaching the unreached & management of TB in hard to reach areas, urban slums, Migrants, etc. Addressing huge reservoir of latent TB infection is yet another challenge which has been taken up by the programme. Strengthening quality of care in private sector is yet another challenge which need to be addressed urgently. Looking forward, newer strategies like LTBI Management, BPaL regimen seems to be the hope to achieve our End TB target by 2025. Along with this, we need to engage leaders from all walks of life: Politics, Faiths, Sports and Arts & also engage social organizations like Rotary, Lions, Development Organizations etc. We should expand our community outreach programmes & Engage communities, patients, and their families to be leaders in the fight against TB The phase that we are in right now, is the most crucial phase to a TB free world and if we don't join hands now, we will have to face huge losses in the coming years. NTEP will have to make this dream come true for the present as well as the future generation.[Table 1]
| Indicators | Total (%) |
|---|---|
| % of target TB notification achieved | 86% |
| % of TB notified patients with known HIV status | 70% |
| % of eligible TB notified patients with UDST done | 71% |
| Treatment Success Rate | 77% |
| % of eligible beneficiaries paid under NikshayPoshan Yojana | 69% |
| % of diagnosed MDR patients initiated on treatment | 80% |
| % of eligible contact children (< 6 years) given chemoprophylaxis | 31% |
| % of eligible PLHIV given IPT | 14% |
| % of expenditure amongst the approved ROP of the State (FY 2019-20) | 8% |
REFERENCES
- Microbe Profile: Mycobacterium tuberculosis: Humanity's deadly microbial foe. Microbiology. 20181;64:437-439.
- [CrossRef] [PubMed] [Google Scholar]
- A Novel Pathogenic Taxon of the Mycobacterium tuberculosis Complex, Canetti: Characterization of an Exceptional Isolate from Africa. International journal of systematic bacteriology. 1997;47(4):1236-45.
- [CrossRef] [PubMed] [Google Scholar]
- Tuberculosis: Epidemiology and Control. Mediterr J Hematol Infect Dis. 2014;6(1):e2014070.
- [CrossRef] [PubMed] [Google Scholar]
- Global tuberculosis control. WHO report WHO/HTM/TB/2006.362. Geneva: World Health Organization, 2006
- [Google Scholar]
- (https://www.who.int/tb/publications/global_report/en/) (accessed )
- https://en.wikipedia.org/wiki/Sushruta_Samhita (accessed )
- Tuberculosis: Current situation, challenges and overview of its control programs in India. J Global Infect Dis. 2011;3:143-50.
- [CrossRef] [PubMed] [Google Scholar]
- The history of Tuberculosis Control in India: Glimpses through decades.Tuberculosis Control in India. New Delhi, India: Directorate General of Health Services, Ministry of Health and Family Welfare. 2005:15-22.
- [Google Scholar]
- http://ntiindia.kar.nic.in/aboutus.htm (accessed )
- New Vision for Revised National Tuberculosis Control Programme (RNTCP): Universal access - "Reaching the un-reached" Indian J Med Res. 20121;35:690-4.
- [Google Scholar]
- xDR-TB: An outcome of programmatic management of TB in India. Indian Journal of Medical Ethics. 2014;11(1):47-52.
- [Google Scholar]
- https://www.nikshay.gov.in/ (accessed )






