Translate this page into:
Assessment of Organophosphorus Pesticide Exposure Through Red Blood Cell Acetylcholinesterase Levels in Blood of Urban Residents
*Corresponding author: Anupa Yadav, Department of Industrial Hygiene, ICMR - Centre for Ageing and Mental Health, Kolkata, West Bengal, India. anupayadav08@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Yadav A, Kumari K, Das S, Chakrabarti A, Chhangte JV. Assessment of Organophosphorus Pesticide Exposure Through Red Blood Cell Acetylcholinesterase Levels in Blood of Urban Residents. J Compr Health. doi: 10.25259/JCH_61_2024
Abstract
Background
Chronic exposure to organophosphorus (OP) pesticides poses serious health risks. India, the largest pesticide producer in Asia, exposes its population to pesticide residues through air, water, and food. Human biomonitoring is a crucial tool for estimating exposure worldwide. OP compounds are potent cholinesterase inhibitors; acetylcholinesterase (AChE) inhibition can lead to symptoms from weakness to fatal respiratory or cardiac failure. Blood AChE assessment is widely used as a biomarker for cholinesterase inhibition due to OP exposure.
Objectives
The objectives of the study are to assess the level of red blood cell (RBC) acetylcholinesterase in urban residents of Kolkata city.
Material and Methods
AChE levels in RBC were measured by the Ellman method. The blood samples were collected through a becton dickinson and company (BD) vacutainer needle into BD vacutainer polystyrene tubes containing K2 ethylenediaminetetraacetic acid. Sociodemographic details of study individuals were collected by questionnaire.
Results
Blood sample was collected from healthy volunteers (n = 25) aged 20–58 years. The study duration was from February to July 2019. The activity of cholinesterase (µmoles/min/mL) ranges from 1.48 to 2.32 (mean 2.0 ± 0.24). 3% of study individuals had cholinesterase <2.0 µmoles/min/mL. AChE and hemoglobin levels showed a moderate positive significant correlation (r = 0.44; P = 0.03) among study individuals. Females (1.71 µmoles/min/mL) have lower AChE than males (2.13 µmoles/min/mL). Levels of AChE were reduced in middle-class individuals than upper middle. AChE levels were more prone to reduction in the older age group.
Conclusion
Study individuals had indirect exposure to pesticides. Reduction in cholinesterase levels may lead to various health effects. Therefore, biomonitoring of pesticide exposure among the general population is also very imperative. AChE enzyme level in RBC may be used as a screening tool for OP pesticide exposure.
Keywords
Pesticides
Cholinesterase activity
Biomonitoring
Urban residents
INTRODUCTION WITH OBJECTIVES
The use of pesticides has been tremendously increased in agriculture products during the past few years. Simultaneously, organochlorines were replaced by organophosphorus (OP) and carbamate pesticides at a distinct rate. These OP compounds interfere with the enzyme acetylcholinesterase (AChE) and inhibit its activities in the nervous system and muscle tissues.1,2 Acute exposure to OP compounds results in the inhibition of AChE activity in the neurological system that may lead to transmission impairment in the respiratory, myocardial, and neuromuscular systems.3,4 Its long-term impacts did not have enough literature, although few reports reveal a reduction in gestation period and reduced birth length due to low exposure to environmental OP compounds.5-7 The General population of all age groups have exposure to these OP-based pesticides indirectly through the food chain. Globally, approximately 3 million individuals are under the influence of OP compounds; among these, about 3 lakhs either pass away or at severe morbidity stage.8 Organophosphate compounds inhibit the activities of the AChE enzyme by phosphorylating serine hydroxyl group present at the active site of this enzyme.9 Cholinesterase is commonly used as a biomarker of acute or chronic exposure screening to OP pesticides.10,11 Assessment of AChE will help to indicate whether a person has exposure to OP pesticides or not. OP compound exposure can cause various biochemical alterations before harmful clinical health effects.12 Biomonitoring of red blood cells (RBCs) AChE activity is a well-documented reliable biomarker for indicator of pesticide exposure.9,10,13 A lot of literature showed an assessment of AChE among exposed occupational groups, and the exposed group had AChE levels lower than their control group.3,10,14,15 Study carried out by Dhananjayan et al. (2012) among pesticide-exposed workers, the AChE mean value was 2.51 µmoles/min/mL in control subjects and 2.91 µmoles/min/mL; their results observed significant 14% reduction in AChE active among exposed group than the control.3
However, the control counterpart of these occupational groups revealed the level of AChE level lower than the normal range (10.229–23.761 µmoles/min/mL, referred to in laboratory analysis).16 Literature reveals a reduction in the activity of the AChE enzyme even among healthy individuals.17 Therefore, in light of the above evidence, the present work was carried out to assess the levels of AChE among urban residents, too.
MATERIAL AND METHODS
Study individuals
The study included 25 healthy urban male and female participants belonging to urban areas employed in a research institute located in Kolkata city. The study includes individuals coming from central Kolkata, North 24 Parganas, South Kolkata, and the Howrah district of West Bengal. The study was conducted from February to July 2019. They did not have direct exposure to any pesticide. However, indirectly, they may have exposure to pesticides through the food chain. Detailed data on their socioeconomic characteristics were collected by a questionnaire. Written informed consent was obtained from all the study participants after explaining the study’s purpose. A routine physical examination was done, including blood pressure, pulse rate, height, and weight of all participants.
Blood analysis
To assess the level of AChE enzyme, four milliliters of venous blood were collected by venipuncture from the cubital vein of fasting subjects in the supine position. The blood was collected through a BD vacutainer needle into BD vacutainer polystyrene tubes containing K2 ethylenediaminetetraacetic acid (BD Franklin, NJ, USA). The activity of AChE was measured by using the Ellman method modified by Chambers and Chambers.3,18,19 Plasma was separated by centrifuging the blood at 2000 rpm for 10 min at 4°C. RBCs were washed with isotonic saline 3 times to remove any remaining plasma. RBCs were lysed by adding an equal volume of cold distilled water to the packed cells and centrifuged again to remove cell debris. 0.2 mL of the hemolysate (supernatant) was mixed with 3 mL of phosphate buffer (0.1 M, pH 7.4) and 0.1 mL of 5,5’-dithiobis (2-Nitrobenzoic acid) solution in a cuvette. 0.1 ml of acetylthiocholine iodide was added to the cuvette to initiate the reaction and immediately, the reaction mixture was mixed by gentle swirling. Sample readings were taken with an ultraviolet-visible spectrophotometer at 412 nm. Automated hematology analyzers were used for the estimation of blood hemoglobin (Hb).
Statistical analysis
Data were tabulated in Microsoft Excel, and statistical analysis was done using the Statistical Package for the Social Sciences software version 21.0. Student t-text was applied to compare mean values of AChE between two groups for different parameters. To find the association between variables, the Spearman rank correlation was applied. Statistical significance was considered when P < 0.05.
RESULTS
The mean age of study individuals was 35 ± 11 years, ranging from 20 to 58 years; 76% were males and 24% were females. The sociodemographic characteristics of study individuals (n = 25) are presented in Table 1. Body mass index (BMI) mean ± standard deviation was 21 ± 3 kg/m2; most of the study individuals were in the normal range of BMI, and only 8% were in the obese category. About 60% of the study individuals were in the upper middle class, and 40% were in the middle class. Most of the study individuals have mixed type dietary habits (i.e., they eat vegetarian and non-vegetarian food such as fish and chicken mainly), and 16% were vegetarians. 80% of them were non-smokers, and alcohol consumption prevalence was 76%.
| Variables | Subjects n (%) |
|---|---|
| Gender | |
| Male | 19 (76) |
| Female | 06 (24) |
| Body mass index (kg/m2) | |
| Normal | 23 (92) |
| Obese | 02 (8) |
| Economic status | |
| Middle | 10 (40) |
| Upper middle | 15 (60) |
| Dietary habit | |
| Vegetarian | 04 (16) |
| Mixed | 21 (84) |
| Smoking | |
| Yes | 05 (20) |
| No | 20 (80) |
| Alcohol consumption | |
| Yes | 06 (24) |
| No | 19 (76) |
BMI: Body mass index: Normal range 18–25 kg/m2, Obese >25 kg/m2
Our results showed that the mean value of AChE levels was 2.0 ± 0.24 µmoles/min/mL, ranging from 1.48 to 2.32 µmoles/min/mL, presented in Table 2. Figure 1 represents gender-wise variation in levels of AChE enzyme, 24% of the study individuals had cholinesterase activity <2.0 µmoles/min/mL, and unfortunately, these individuals represent females only, whereas all the males have AChE levels >2.0 µmoles/min/mL. However, this difference in the level of AChE enzyme between males and females was not statistically significant (P> 0.05).
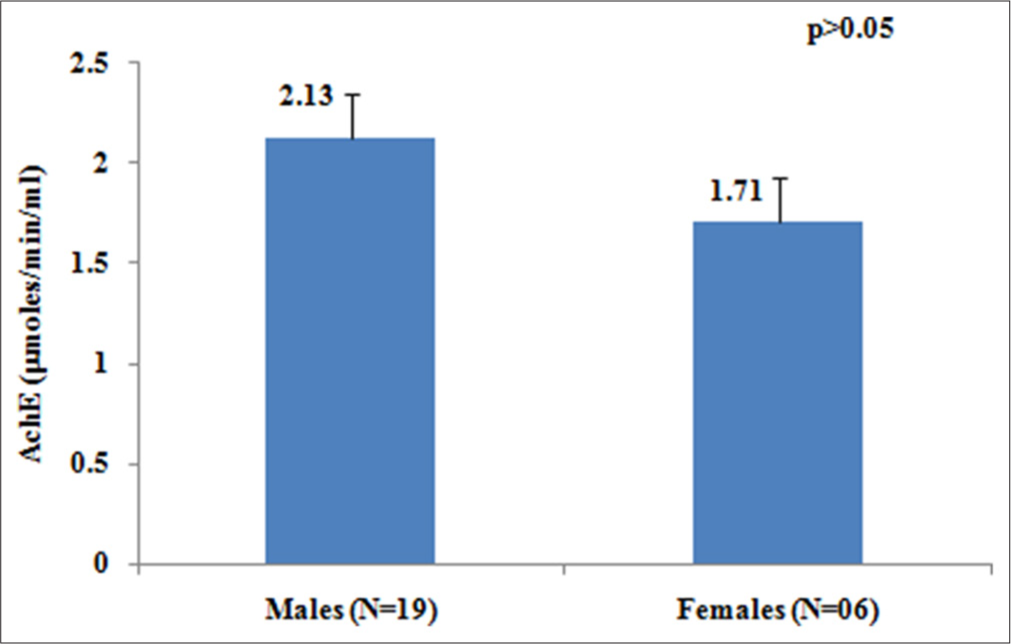
- Gender-wise variation in acetylcholinesterase (µmoles/min/mL) levels in study individuals.
| Study Individuals | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Total (n=25) | 2.03 | 0.22 | 1.48 | 2.32 |
| Males (n=19) | 2.13 | 0.22 | 2.01 | 2.32 |
| Females (n=06) | 1.71 | 0.24 | 1.48 | 1.89 |
RBC: Red blood cell, AChE: Acetylcholinesterase, SD: Standard deviation
Individual economic status plays a very crucial role when considering the impact of pesticide exposure on the activity of enzymes that are involved in neurotransmitter activities in humans. Therefore, we categorized the study individuals, as per the revised, modified BG Prasad classification for October 2023, into two categories, middle class, and upper middle, based on their per capita monthly income to find the variation in the level of AchE enzyme.20 We found that the level of AChE enzyme was lower in middle-class individuals (1.98 µmoles/min/mL) when compared with the upper middle class (2.06 µmoles/min/mL), as presented in Figure 2. However, this variation in AChE levels was statistically not significant (P> 0.05).
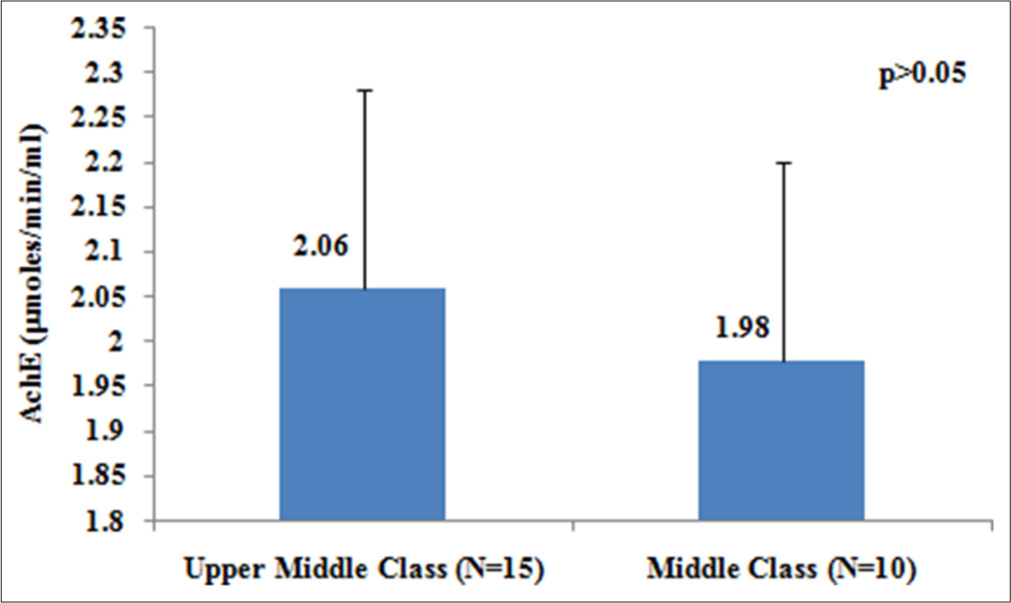
- Variation in acetylcholinesterase (µmoles/min/mL) levels with economic status of study individuals.
When the impact of age group was assessed on AChE enzyme level, it was lower (1.98 µmoles/min/mL) in individuals of age >37 years than that of <37 years (2.06 µmoles/min/mL). However, this difference was not significant. No significant variation in AChE enzyme levels was observed between smokers and non-smokers, or between alcoholic and non-alcoholic individuals. The mean value of Hb level in our study individuals was 13.05 ± 1.15 g/dL. Among 12% of the total study individuals, the Hb level was less than the normal range (12–17.4 g/dL). AChE level showed a moderate positive significant correlation (r = 0.44; P = 0.03) with Hb levels among study individuals, as shown in Figure 3. As per the World Health Organization (WHO) (2011), the anemic condition has three categories based on Hb level: Mild (11– 12.9 g/dL), moderate (8–10.9 g/dL), and severe (<8.0 g/dL) anemia.21 Therefore, according to this classification, 36% of the individuals were mild anemic, and none of the individuals had moderate and severe anemia.
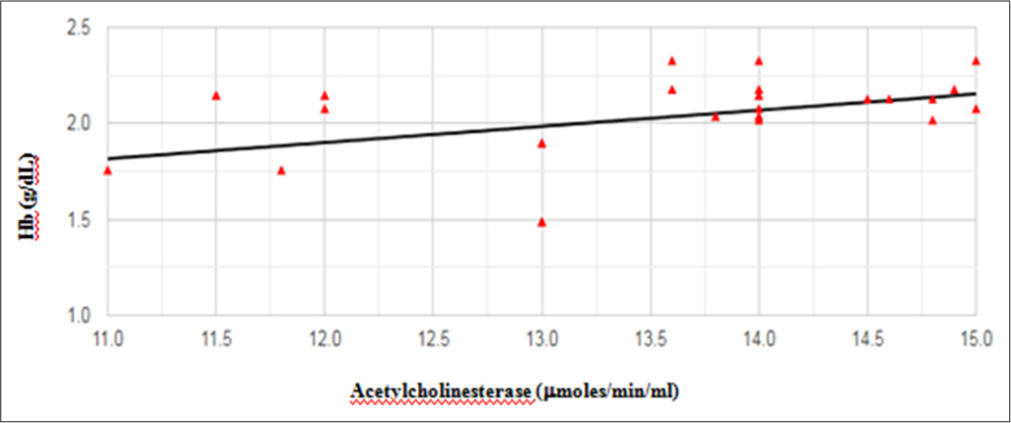
- Correlation between hemoglobin and acetylcholinesterase levels among study individuals (n=25).
DISCUSSION
The present work was carried out to assess the levels of RBC AChE enzyme and Hb concentrations among urban residents of Kolkata city from a selected group. The impact of socioeconomic factors such as gender, age, and economic status on levels of AChE enzyme was also evaluated. The study individuals were comprised of both male and female individuals. They all work in a research institute at various designations, such as researchers, administration clerks, stenographers, technicians, canteen cooks, gardeners, sweepers, and security guards. They did not have any direct exposure to OP compounds and enough variation in their economic status and lifestyle. Therefore, this study group was well suited for the assessment of indirect exposure to OP compound and variation in levels of AChE enzyme.
Females (1.71 µmoles/min/mL) have lower levels of AChE than males (2.13 µmoles/min/mL). Levels of AChE were reduced in middle-class individuals than in the upper middle. AChE levels were more prone to reduction in the older age group. Our results revealed that AChE levels ranged from 1.48 to 2.32 µmoles/min/mL, with a mean value of 2.0 µmoles/min/mL. The mean AChE level in our study was lower than that observed in control subjects reported by Dhananjayan et al. (2012).3 Mohamed et al. (2022) found that the mean AChE level was lower among rural subjects (3.8 µmoles/min/mL) compared to urban subjects (7.7 µmoles/min/mL), with a reduction of approximately two-fold.10 This decrease was attributed to greater pesticide exposure among rural residents due to agricultural activities. The mean AChE level observed in our study was about 2 times lower than that of the urban residents reported by Mohamed et al. (2022).10 According to the WHO report, OP poisoning affects millions of people worldwide each year.22 OP compounds play a critical role in agriculture, making exposure to these chemicals almost unavoidable. The reference values for erythrocyte cholinesterase, as recommended by the laboratory in the study by Mattiazzi et al. (2020), are 10,229–23,761 U/L (or 10.229-23.761 µmoles/min/mL).16 Our findings show erythrocyte cholinesterase levels approximately 5 times lower than the reference values used by Mattiazzi et al. (2020), strongly indicating indirect exposure to OP compounds in our study individuals.16
A study by Rathish et al. (2018) involving 100 healthy, unexposed urban residents found a mean AChE activity of 449.8 mU/µM Hb, with Hb levels ranging from 18.0 to 41.5 U/g Hb. Based on the mean AChE activity (449.8 mU/µM Hb) and the lowest Hb value (18.0 U/g Hb), the converted AChE activity in µmoles/min/mL was 1.25.23 This study demonstrated that AChE activity is reduced even among healthy individuals with low-level, indirect exposure to OP pesticides.23 Similarly, our results also indicate decreased AChE levels among urban residents despite the absence of direct OP exposure. Supporting this finding, a toxicological study from the Western Province of Sri Lanka also reported reduced AChE activity among healthy participants.17 Moreover, a study from Pakistan found significantly lower AChE levels in an unexposed group (10.8 µmoles/min/mL) compared to an exposed group (3.8 µmoles/min/mL).24
Our results revealed a significant positive correlation between AChE enzyme activity and Hb levels among the study participants, indicating that an increase in Hb levels may enhance AChE activity. These findings align with those of Mohamed et al. (2022), Wafa et al. (2013), and Fareed et al. (2013).10,25,26 Similarly, Khan et al. (2023) observed a notable reduction in Hb levels in an OP-exposed group compared to a control group, with mean Hb levels of 12 ± 1.89 g/dL in the exposed group and 14.9 ± 1.34 g/dL in the control group.24 In their study, 10.36% of the non-exposed group had Hb values below the normal range, while in our study, approximately 12% of individuals showed Hb levels below normal. In addition, our findings revealed that around 20% of the participants had Hb levels under 12 g/dL, a level seen in the OP-exposed group in Khan et al. (2023).24 This suggests that these individuals in our study might have had higher indirect pesticide exposure than others, though other underlying factors may also contribute to their lower Hb levels. The prevalence of mild anemia among our study participants was 36%.
Limitations of the study
This study was conducted as part of an academic dissertation, which limited the sample size of participants. Despite this, the findings offer valuable insights into the effects of low-level environmental exposure to OP compounds among urban residents without occupational exposure. These results emphasize the need for further biomonitoring studies on OP compounds in a larger, general population.
CONCLUSION
Our results highlight a decrease in AChE activity even among healthy, unexposed individuals. Females appear to be at greater risk than males, and older individuals are more susceptible to reductions in AChE levels than younger individuals. Prolonged, low-level exposure could potentially contribute to undetected chronic poisoning. While a comprehensive comparison was limited by the lack of similar studies at the local, national, and international levels, this work provides valuable data on AChE activity among occupationally unexposed, healthy urban residents.
Ethical approval
The research/study was ethically approved by Institutional Ethical Committee, approval number 9th IEC/ROHC (E)/5.7, dated 16 January 2015.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: Nil.
References
- Comparing the Organophosphorous and Carbamate Insecticide Resistance Mutations in Cholin-and Carboxyl-esterases. Chem Biol Interact. 2005;15:157-8.
- [CrossRef] [PubMed] [Google Scholar]
- Common Mechanism of Toxicity: A Case Study of Organophosphorus Pesticides. Toxicol Sci. 1998;41:8-20.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of Acetylcholinesterase and Butyrylcholinesterase Activities in Blood Plasma of Agriculture Workers. Indian J Occup Environ Med. 2012;16:127-30.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic Significance of Estimation of Pseudocholinesterase Activity and Role of Pralidoxime Therapy in Organophosphorous Poisoning. Toxicol Int. 2013;20:214-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of Transplacental Exposure to Environmental Pollutants on Birth Outcomes in a Multiethnic Population. Environ Health Perspect. 2003;111:201-5.
- [CrossRef] [PubMed] [Google Scholar]
- Prenatal Insecticide Exposures and Birth Weight and Length among an Urban Minority Cohort. Environ Health Perspect. 2004;112:1125-32.
- [CrossRef] [PubMed] [Google Scholar]
- In Utero Pesticide Exposure, Maternal Paraoxonase Activity, and Head Circumference. Environ Health Perspect. 2004;112:388-91.
- [CrossRef] [PubMed] [Google Scholar]
- The Role of Oximes in the Management of Organophosphorus Pesticide Poisoning. Toxicol Rev. 2003;22:165-90.
- [CrossRef] [PubMed] [Google Scholar]
- Structure and Mode of Action of Organophosphate Pesticides: A Computational Study. Comput Theor Chem. 2016;1088:9-23.
- [CrossRef] [Google Scholar]
- A Comparative Study: Rural Versus Urban Children as Regard Exposure to Organophosphorus Pesticides Using Cholinesterase Enzyme Activity. Beni-Suef Univ J Basic Appl Sci. 2022;11:6.
- [CrossRef] [Google Scholar]
- Adverse Impact of Insecticides on the Health of Palestinian Farm Workers in the Gaza Strip: A Hematologic Biomarker Study. Int J Occup Environ Health. 2005;11:144-9.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of Exposure to Pesticides on Serum Components and Enzyme Activities of Cytotoxicity among Intensive Agriculture Farmers. Environ Res. 2006;102:70-6.
- [CrossRef] [PubMed] [Google Scholar]
- Joint Action of Combinations of Pollutants on the Acetylcholinesterase Activity of Several Marine Species. Ecotoxicology. 1995;4:266-79.
- [CrossRef] [PubMed] [Google Scholar]
- Pesticide Dose Estimates for Children of Iowa Farmers and Non-farmers. Environ Res. 2007;105:307-15.
- [CrossRef] [PubMed] [Google Scholar]
- Pesticide Exposure of Children in an Agricultural Community: Evidence of Household Proximity to Farmland and Take Home Exposure Pathways. Environ Res. 2000;84:290-302.
- [CrossRef] [PubMed] [Google Scholar]
- Hearing Screening and Cholinesterase Activity among Rural Workers Exposed to Pesticides. Rev Brasil Med Trabalho. 2020;17:239.
- [CrossRef] [PubMed] [Google Scholar]
- Exposure to Acetylcholinesterase-inhibiting Pesticides and Chronic Renal Failure. Ceylon Med J. 2006;51:42-3.
- [CrossRef] [PubMed] [Google Scholar]
- A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem Pharmacol. 1961;7:88-95.
- [CrossRef] [PubMed] [Google Scholar]
- An Investigation of Acetylcholinesterase Ibhibition and Aging and Choline Acetyltransferease Activity Following a High Level Acute Exposure to Paraoxon. Pest Biochem Physiol. 1989;33:125-31.
- [CrossRef] [Google Scholar]
- Updated Modified BG Prasad Classification for October 2023. Natl J Community Med. 2024;15:89-90.
- [CrossRef] [Google Scholar]
- Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity Geneva: World Health Organization; 2011.
- [Google Scholar]
- Novel Clinical Toxicology and Pharmacology of Organophosphorus Insecticide Self-poisoning. Annu Rev Pharmacol Toxicol. 2019;59:341-60.
- [CrossRef] [PubMed] [Google Scholar]
- Red Blood Cell Acetylcholinesterase Activity among Healthy Dwellers of an Agrarian Region in Sri Lanka: A Descriptive Cross-sectional Study. Environ Health Prev Med. 2018;23:25.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of Chronic Exposure of Organophosphorus Pesticide on Hematological and Biochemical Parameters of Agriculture Workers: A Cross Sectional Study. J Pharm Negative Results. 2023;14:3059-67.
- [Google Scholar]
- Oxidative Stress, Hematological and Biochemical Alterations in Farmers Exposed to Pesticides. J Environ Sci Health B. 2013;48:1058-69.
- [CrossRef] [PubMed] [Google Scholar]
- Adverse Respiratory Health and Hematological Alterations among Agricultural Workers Occupationally Exposed to Organophosphate Pesticides: A Cross-sectional Study in North India. PLoS One. 2013;8:e69755.
- [CrossRef] [PubMed] [Google Scholar]






