Translate this page into:
Assessment of Antibody levels amongst Health Care Workers vaccinated with two doses of COVID vaccine 6 months ago in a Hospital of Kolkata, West Bengal: Is there any Need for a Third dose (Booster Dose) of COVID Vaccine?
CORRESPONDING AUTHOR: Dr. Debdutta Haldar, Assistant Professor, Community Medicine, Diamond Harbour Medical College, Kolkata E Mail ID: debduttahaldar@gmail.com
-
Received: ,
Accepted: ,
CITATION: Dhar T, Kole S, Dey A, Haldar D, Sinha S, Choudhury NA. Assessment of Antibody levels amongst Health Care Workers vaccinated with two doses of COVID vaccine 6 months ago in a Hospital of Kolkata, West Bengal: Is there any Need for a Third dose (Booster Dose) of COVID Vaccine?J Comp Health. 2022;10(1):31-36. Doi: https://doi.org/10.53553/JCH.v10i01.005
Abstract
Background:
Vaccines can prevent the adverse effects, even if the virus is contracted. While data shows that two vaccine shots are largely effective at protecting against severe Covid-19, data on the benefit of booster shots is currently unclear.
Objectives:
To the clinical exposure profile of the doctors who had taken both doses of vaccines 6months and before from COVID vaccination centre of BelleVue Clinic, Kolkata and to analyse the antibody levels of the doctors who had been vaccinated with two doses of vaccine 6months ago and before.
Methodology:
An observational, descriptive, cross-sectional, hospital based study was conducted in the Bellevue Clinic, Kolkata. A total of 130 health care workers including doctors, nurses, housekeeping and administrators gave consent to participate. Blood was collected from them and antibody test was conducted by COVID specific antibody testing kits.
Results:
The results showed 80% of the persons whose titre came below the cutoff mark were males. The health care workers belonging to age group 40-60 years were the maximum having lower antibody titres. Doctors were the persons who were mostly having lower levels of antibody titres.
Conclusion:
The study opened up a wide angle to the antibody levels of the covid vaccinated health care workers.
Keywords
COVID Vaccine
COVID 19 Antibody
Healthcare worker.
INTRODUCTION
In the COVID-19 pandemic, vaccination has been very effective in combatting with the disease burden. Acquiring immunity against the virus is essential, which can be acquired in two ways, either naturally or through vaccine. Vaccines can prevent the adverse effects, even if the virus is contracted. The COVID-19 vaccination in India began from 16 January, 2021[1]. The first group to receive the doses included the healthcare workers. A separate booster program against Covid-19 would aim to extend protection for millions more at high risk from the virus, although there is disagreement over whether this is really necessary.[9] While data shows that two vaccine shots are largely effective at protecting against severe Covid-19, data on the benefit of booster shots is currently unclear. To establish whether a booster dose would offer significantly increased protection, experts first need to understand the level of antibodies and other immune defences needed to prevent severe illness and death from Covid-19. Once this has been measured, and immunity is seen dropping to this level, a case for routinely administering booster shots can be made. A recent Israeli study found a third dose of Pfizer's vaccine to be 86% effective in people aged over 60, and a UK trial assessing whether a third shot of different Covid-19 vaccines could boost immunity against the virus is ongoing, with results expected soon. Preliminary data from an Oxford University-led study evaluating the AstraZeneca vaccine has also found that third dose of the jab boosts antibodies against Covid-19.[10] As per FDA fact sheet, for the use of "high-titre” convalescent plasma, is defined by a neutralizing antibody titre of ≥250 AU/ml in the Broad Institute's neutralizing antibody assay or an S/C cut-off of ≥12 AU/ml by the Ortho VITROS IgG assay against “S” protein [11] thus this S/C cut-off of ≥12 AU/ml appears significant in providing adequate neutralization against SARS Cov-2 virus. In this context S/C cut-off of < 12 AU/ml could be considered as waning antibody (neutralizing) response Since there are data showing relation between lower antibody levels and contraction of Covid 19 disease we would take a cut off specific for the antibody kit we are using to determine the lower antibody levels. The analysis of the convalescent plasma expanded access program suggests the most benefit is seen when convalescent plasma is given in the first three days from diagnosis. [12] Hence, based on all the available data, we would like • to conduct a study to see the clinical exposure profile of the doctors who had taken both doses of vaccines 6months and before from COVID vaccination centre of Belle Vue Clinic, Kolkata • to analyse the antibody levels of the doctors who had been vaccinated with two doses of vaccine 6 months ago and before.
MATERIAL & METHODS
Study Design:
Observational, descriptive
Study Type:
cross sectional, hospital based study
Study Setting:
It was conducted in the pathology department of Belle vue Clinic of Kolkata, West Bengal
Study Population:
The health care workers who had taken the second dose of vaccine at least 6 months ago were taken as study participants. Study Duration : 1year(June 2021-May 2022)
Sampling design/technique:
Complete enumeration of all the healthcare workers who had taken both doses of vaccines at least 6 months before from CVC of Belle Vue clinic were taken as the sample.
Study tools:
Pre designed data sheet, hospital register, Beckman coulter dxi 800 COVID specific antibody kits. (IgG SARS Cov-2 antibody against ‘S’ protein)
Inclusion criteria:
All the healthcare workers who had taken both doses of vaccines at least 6 months before from CVC of Belle Vue clinic. Those healthcare workers willing to participate in the study and gave consent for the same.
Exclusion criteria:
Those Healthcare workers, who are:
-Non vaccinated
-Partially vaccinated
-Completely vaccinated but less than 6 months ago
-Not giving consent to participate
-Severely ill
Study procedure/Strategy for data collection:
After taking ethical clearance from the Institutional Ethical Committee of Belle Vue Clinic, all the Healthcare workers who fulfilled the inclusion criteria were noted from the hospital registers. The persons were called for a antibody testing on prefixed scheduled dates. The persons who gave consent were provided with predesigned validated questionnaire. A total of 130 health care workers including doctors, nurses, housekeeping, administrators gave consent to participate. The biological sample consent was also taken.
Blood was collected from them and antibody test was conducted by COVID specific antibody testing kits (PCroduct V 74339 -Access S variants ARS CoV -2 IgG (1st IS)Reagent Kit (200 tests/kit). The test results were obtained and the combined data were taken in Microsoft excel sheets.
Standard operating Definitions Antibody titre(negative):
As per FDA fact sheet, for the use of “high-titre” convalescent plasma, is defined by a neutralizing antibody titre of ≥250 AU/ml in the Broad Institute's neutralizing antibody assay or an S/C cut-off of ≥12 AU/ml by the Ortho VITROS IgG assay against “S” protein [11] thus this S/C cut-off of ≥12 AU/ml appears significant in providing adequate neutralization against SARS Cov-2 virus. In this context S/C cut-off of < 12 AU/ml could be considered as waning antibody (neutralizing) response.
Data analysis:
All collected data were analysed by suitable and appropriate software(Microsoft Excel) and statistical techniques.
RESULTS
The study was carried out the Pathology department of Belle Vue Clinic, Kolkata. The data was analysed and results were obtained This may be due to the maximum exposure to the Covid patients that their antibody titre weaned off early.
after two doses of vaccinations only 34% had contracted Covid in the last 6months and their antibody levels were found to be higher than those who did not have Covid infection.
Table 1 shows out of the 130 study participants, 60% were male and 80% of the persons whose titre came below the cut-off mark were males. The rationality of male having more negative titres can be that they were more clinically exposed(majority of doctors who participated were male). It is seen from Table 2, that the health care workers belonging to age group 40-60 years were the maximum having lower antibody titres followed by those above 60 years of age.
| Gender | Antibody Titre (Negative) | |
|---|---|---|
| 1 Male | 78(60%) | 32(80%) |
| 2Female | 52 (40%) | 8(20%) |
| Total | 130 | 40 |
| Age Groups(yrs) | Antibody Titre (Negative) |
|---|---|
| 21-40 | 3 |
| 40-60 | 20 |
| >61 | 17 |
| Total | 40 |
The health care workers were further classified into 3 categories i) Directly dealing with patients ii)not handling patients iii) stringent PPE users. The average antibody levels for these category of workers were calculated and represented in fig 3 by line diagram. This clearly shows that those who had direct exposure to patients had much higher antibody titres.
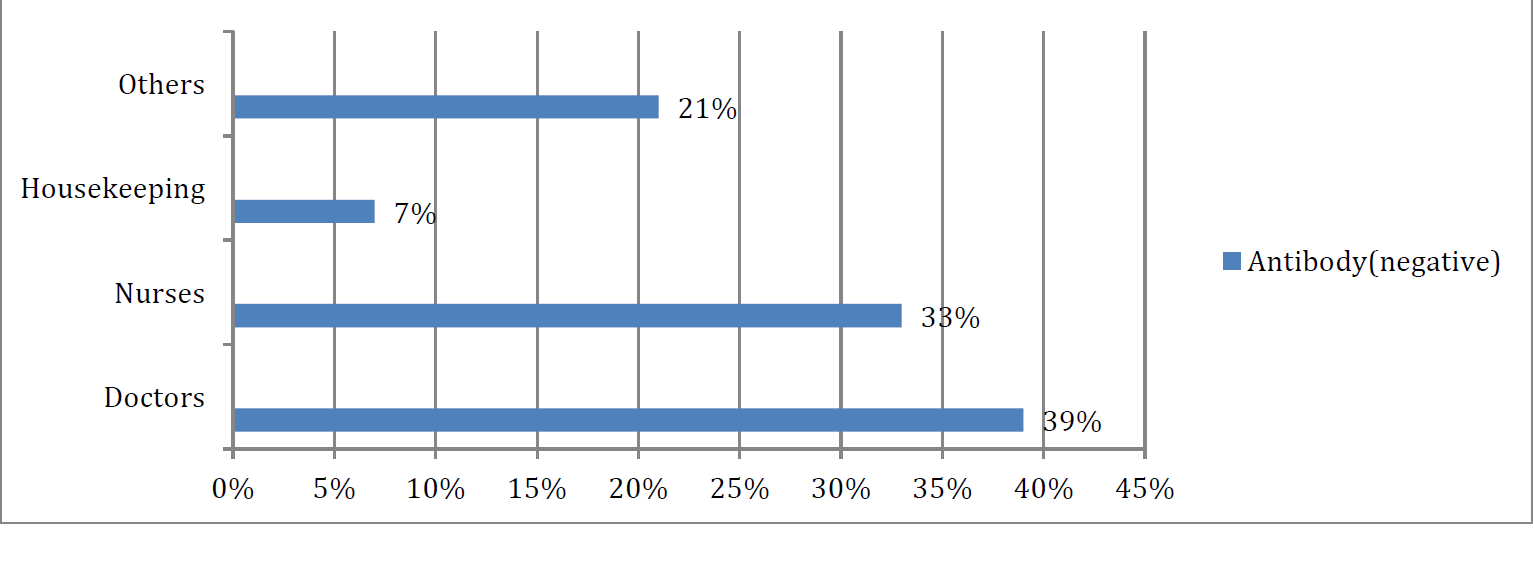
- DISTRIBUTION OF POPULATION ACCORDING TO CATEGORY OF HCWS AND THEIR ANTIBODY TITRE (N=130)
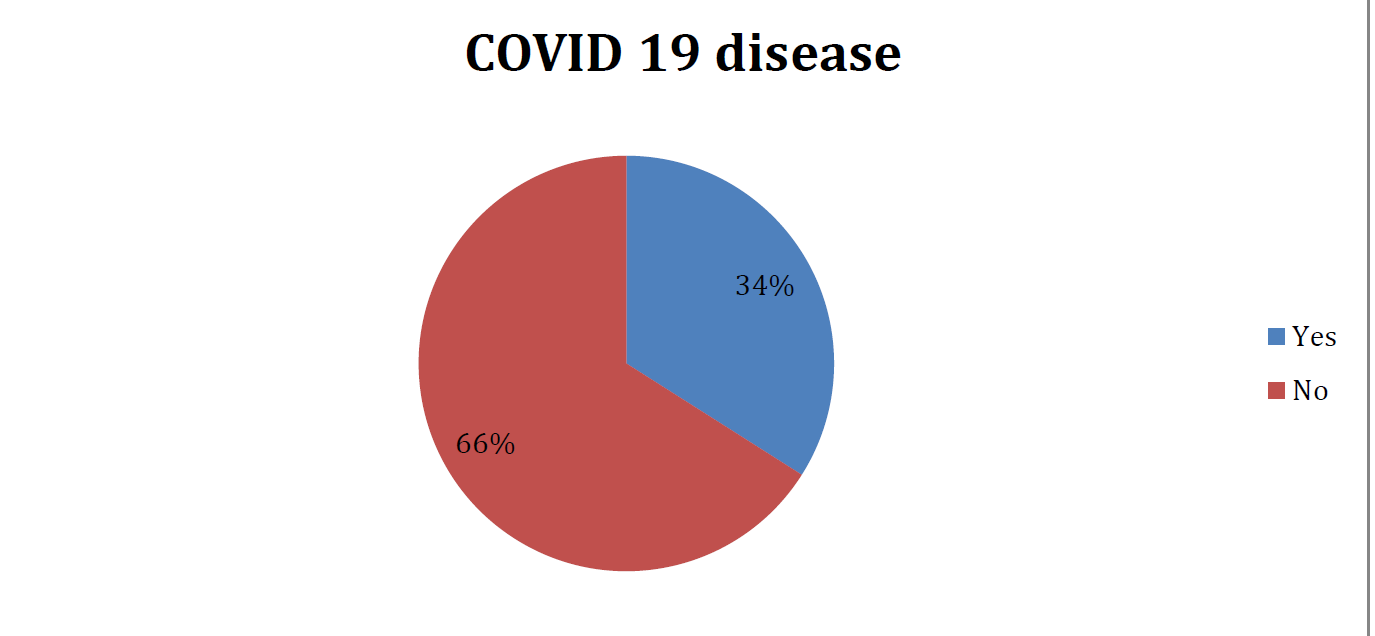
- DISTRIBUTION OF POPULATION ACCORDING TO CONTRACTION OF COVID 19 DISEASE POST VACCINATION(N=130)
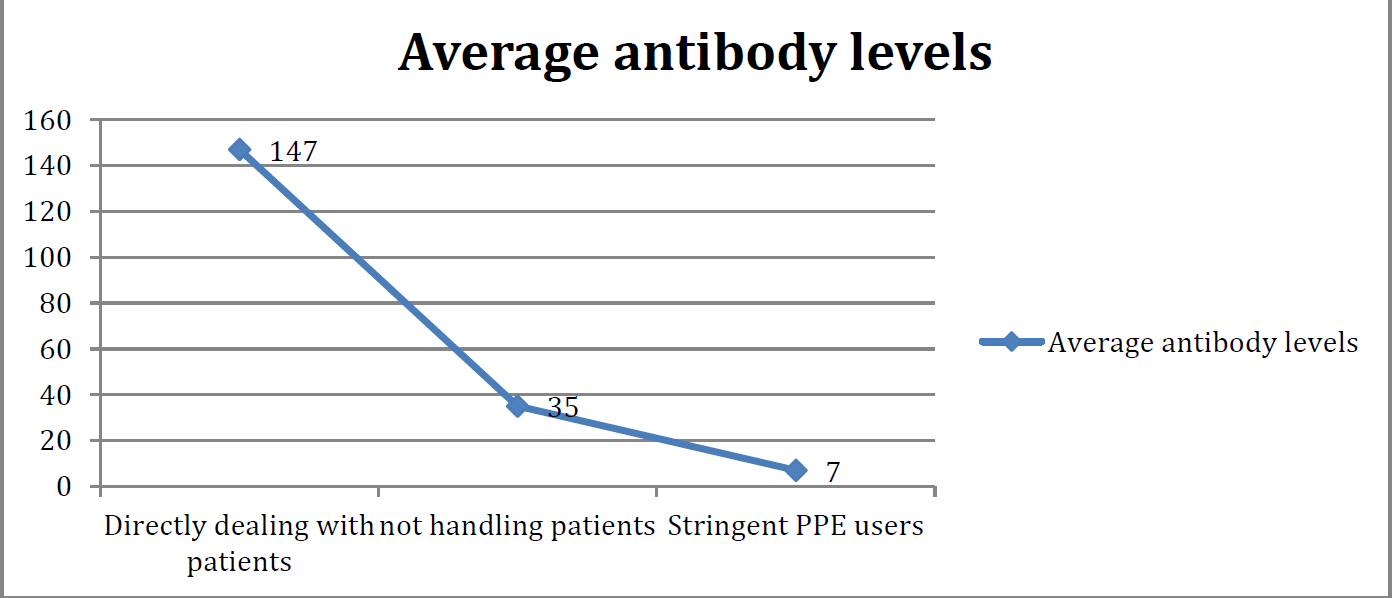
- DISTRIBUTION OF POPULATION ACCORDING TO NATURE OF EXPOSURE TO COVID CASES.
Hence we can see that there was weaning of antibodies in 6 months for those who didnot contract Covid during this span of time. Hence, booster dose definitely have some role to play in those and also protect from any severity of the disease.
DISCUSSIONS
Although the detection of the antibody response to SARS-CoV-2 provides important data to learn whether people have previously been infected with this infection, to diagnose the infection, and to determine the effectiveness of the vaccine, [13] it is also important to identify the factors that affect the variability of this antibody response, understand the pathogenesis of COVID-19, carry out epidemiological studies, and contribute to studies on vaccines. [14]However, the serological response can affect the quality of the immune response and the duration of immunity due to both individual characteristics and vaccine-related differences. [15]
Antibodies that arise as a response to humoral immunity in individuals previously infected with or vaccinated against COVID-19 are present in the blood for a certain period of time. Although antibodies produced by short-lived plasma cells in secondary lymphoid organs increase rapidly in the blood and then decrease rapidly in the first 3 months, the antibodies produced by long-lived plasma cells in the bone marrow tend to decrease more slowly in the following period.[16]. Although the tendency of the antibody response to decreasing over time is a natural process of humoral immunity, the course of the post-vaccine antibody response is crucial in the current pandemic period. The post-vaccine humoral immune responseshowed seropositivity of 89.7% after the 2nd week, in the study of Tanriöver et al. [17]While in the study of Bayram et al., [18] a sero-positivity of 99.6 was observed 4 weeks after the vaccine. In parallel with the natural course of the antibody response in terms of post-vaccine antibody responses, it was observed to decline in the current study. A decrease in antibody titer was observed at the 12th week of the inactive whole virion vaccine in the participants who had 75-250 U/ml antibodies after excluding those with >250 U/ml antibodies. Although there was a statistically significant decrease in antibody titers, it was observed that the sero-positivity of the participants still continued. Evaluating the mRNA post-vaccine antibody titer, Naaber et al. also observed that the RBD antibody response obtained six weeks following the vaccine was decreased compared to the antibody response observed one week after the vaccine. [19]
Although there is not enough postvaccine data on NCP antibodies, [20] which can be detected in the blood even eight months after infection by COVID-19 [21] and are quite specific in the diagnosis of COVID-19 infection, the NCP antibody response was measured as negative in three separate studies in which the NCP antibody levels were measured after an mRNA vaccine targeting only spike protein's RBD. [22], [23] In a study carried out after an mRNA vaccine, the fact that NCP antibodies were negative and antibody response to spike proteins was positive has been evaluated as evidence of the vaccine. [23] In the study, carried out after the CoronaVac vaccine using inactivated virus, most of the participants were found to have a positive antibody response to NCP.The limitation of the study is that the participants did not reflect the general population, as the study was conducted in a small group of healthy volunteers. Therefore, we could not adequately examine the differences in antibody levels in the presence of various diseases that are more likely to be present in the population with the demographic data we examined. In the study, we examined post-vaccine antibody titers. However, the neutralization test, which is an important aspect of the immune system and an indicator of cellular immunity and cellular response, could not be evaluated. The study opened up a wide angle to the antibody levels of the Covid vaccinated health care workers. The results will help the future researchers to develop different means for a Covid free or a normal healthy life based on this data.
LIMITATIONS
The study was done in a hospital setting among health care workers so a bigger population couldnot be taken.
Dr Tirthankar Dutta have arranged for the testing Kits, Dr Saurabh Kole helped in the data Collection along with Dr Nasreen Chowdhury and Dr Sawansikta Sinha. Dr Arijit dey and Dr Debdutta Haldar helped in the analysis.
REFERENCES
- WHO Director-General's opening remarks at the media briefing on COVID-19 -11 March, 2020. (accessed )
- [Google Scholar]
- Ministry of Health and Family Welfare(MoHFW), vaccine registered, Frequently Asked Questions. (accessed )
- [Google Scholar]
- Ministry of Health and Family Welfare The National Regulator grants Permission for Restricted Use in Emergency Situations to Sputnik-V vaccine Posted On: 13 APR 2021 11:56AM by PIB Delhi. PRID=1711342 (accessed )
- [Google Scholar]
- SERUM INSTITUTE OF INDIA PVT. LTD. 212/2, Hadapsar, Pune 411028 India.Coronavirus disease (COVID-19): Vaccines. ChAdOx1 nCoV-19 Corona Virus Vaccine (Recombinant) COVISHIELD™. Available:https://www.seruminstitute.com/product_cov ishield.php (accessed )
- [Google Scholar]
- Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study.
- [Google Scholar]
- (accessed )
- (accessed )
- (accessed )
- (accessed )
- Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin Chim Acta. 2021;20(519):60-63. 10.1016/j.cca.2021.04.006 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- [CrossRef] [PubMed] [Google Scholar]
- The Nucleocapsid protein triggers the main humoral immune response in COVID-19 patients. BiochemBiophys Res Commun. 2021;5(543):45-49. 10.1016/j.bbrc.2021.01.073 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- [CrossRef] [PubMed] [Google Scholar]
- Factors That Influence the Immune Response to Vaccination. Clin Microbiol Rev. 2019;13(32):e00084-18. 2 10.1128/CMR.00084-18 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- [CrossRef] [PubMed] [Google Scholar]
- Serological reconstruction of COVID-19 epidemics through analysis of antibody kinetics to SARS-CoV-2 proteins. medRxiv 10.1101/2021.03.04.21252532 [CrossRef] [Google Scholar]
- [Google Scholar]
- Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (Coronavac): interim results of a double blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213-222. 10.1016/S0140-6736(21)01429-X [PMC free article] [PubMed] [Google Scholar]
- [Google Scholar]
- Quantitation of antibodies against SARS-CoV-2 spike proteinafter two doses of CoronaVac in healthcare workers. J Med Virol. 2021;93:5560-5567. 10.1002/jmv.27098 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- [CrossRef] [PubMed] [Google Scholar]
- Antibody response after COVID-19 mRNA vaccination in relation to age, sex, and side effects. medRxiv 10.1101/2021.04.19.21255714 [CrossRef] [Google Scholar]
- [Google Scholar]
- Immunogenicity and crossreactivity of antibodies to the nucleocapsid protein of SARS-CoV-2: utility and limitations in seroprevalence and immunity studies. Transl Res. 2021;232:60-74.
- [CrossRef] [PubMed] [Google Scholar]
- Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J Clin Virol. 2021;202(136):104765. 10.1016/j.jcv.2021.104765 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- [CrossRef] [PubMed] [Google Scholar]
- Antibodies against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) in individuals with and without COVID-19 vaccination: A method comparison of two different commercially available serological assays from the same manufacturer. Clin Chim Acta. 2021;202(518):9-16. 10.1016/j.cca.2021.03.007 [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- [CrossRef] [PubMed] [Google Scholar]
- Measurement of multiple SARS-CoV-2 antibody titer after vaccination represents individual vaccine response and contributes to individually appropriate vaccination schedules. medRxiv 10.1101/2021.05.21.21257575 [CrossRef] [Google Scholar]
- [Google Scholar]






