Translate this page into:
A Study on the Magnitude of Non-Fermenter Gram-Negative Bacilli and their Antibiotic Susceptibility Pattern in Clinical Samples from a Tertiary Care Hospital, Kolkata
*Corresponding author: Swagata Ganguly Bhattacharjee, Department of Microbiology, Nil Ratan Sircar Medical College and Hospital, Kolkata, West Bengal, India. swagatamedicine@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Choudhury K, Kundu S, Bhunia S, Mallick T, Ganguly Bhattacharjee S, Dey JB. A Study on the Magnitude of Non-Fermenter Gram-Negative Bacilli and their Antibiotic Susceptibility Pattern in Clinical Samples from a Tertiary Care Hospital, Kolkata. J Compr Health. 2024;12:39-43. doi: 10.25259/JCH_4_2023
Abstract
Background:
Non-fermenting Gram-negative bacilli (NFGNB) were initially considered non-pathogenic contaminants but now have emerged as major pathogens because of increased isolation from clinical samples and emerging resistance to common antibacterial agents.
Objectives:
The study aims to determine the magnitude of NFGNB in clinical samples received at the Department of Microbiology, Nil Ratan Sircar Medical College, Kolkata, for a period of one year.
Material and Methods:
The samples were processed according to standard procedures; for identification and antibiotic susceptibility test, Vitek 2 Compact (Biomerieux©) was used.
Results:
Out of 725 bacterial isolates, 169 (23.31%) NFGNB were identified during the entire study period. Pseudomonas spp. (n = 84) was isolated most frequently, followed by Acinetobacter spp. (n = 73), Burkholderia cepacia (n = 7), and Stenotrophomonas maltophilia (n = 5). Both Pseudomonas spp. and Acinetobacter spp. showed greater susceptibility to piperacillin-tazobactam and poor susceptibility against cefepime.
Conclusion:
It is necessary to identify these drug resistant pathogens in healthcare setup. The antibiotic susceptibility pattern of these pathogens needs to be monitored to guide clinicians in better patient care and management by initiating proper antibiotic therapy.
Keywords
Infection control
Acinetobacter infections
Pseudomonas aeruginosa
Cross-infection
Drug resistance
INTRODUCTION WITH OBJECTIVES
Organisms that are aerobic, non-spore-forming, and Gram-negative rod and either do not take carbohydrates as their energy source or utilize them by various metabolic pathways except fermentation are known as Non-fermenting Gram-negative bacilli (NFGNB).1 They are saprophytic in nature but can cause significant infections particularly healthcare-associated infection (HCAI) in hospitalized patients, immunocompromised hosts, for example, patients with hematological malignancies.2 NFGNB are known to account for about 15% of all bacterial isolates from a clinical microbiology laboratory.3 These organisms are intrinsically resistant to commonly used disinfectants and their tendency to colonize on various surfaces have been pivotal in their emergence as important pathogens of HCAI.4 At present, Pseudomonas spp. and Acinetobacter spp. are the most commonly isolated non-fermenters which are pathogenic to humans. Infections caused by other species of NFGNB,e.g., Burkholderia cepacia and Stenotrophomonas maltophilia, are relatively infrequent.5,6 Majority of infections caused by these organisms are secondary to patients’ pre-existing conditions, e.g., burns, prolonged antimicrobial therapy, immunosuppressive therapy, and old age, etc.7 Recent studies have shown that Pseudomonas aeruginosa is the second most common cause of HCAI pneumonia and ventilator-associated pneumonia.8,9 NFGNB shows resistance to a wide range of commonly used antibiotics by several mechanisms, e.g., by producing beta-lactamases, efflux pumps mediated, and penicillin-binding protein site mutation.10
Due to the increased isolation of NFGNB and their resistance against common antibacterial agents, this study was designed to determine the magnitude of NFGNB in clinical samples and their susceptibility pattern in our hospital.
Aims and objectives
The objectives of the study were as follows:
To determine the magnitude of NFGNB among all clinical isolates
To determine the antibiotic susceptibility pattern of NFGNB isolated from clinical samples.
MATERIAL AND METHODS
The study was conducted for a period of one year between January 1 and December 31, 2020, in the Department of Microbiology, Nil Ratan Sircar Medical College, Kolkata. The samples received at the Department of Microbiology from various wards, intensive care unit and outdoors were processed. The samples received for processing were blood, body fluids, urine, sputum, endotracheal tube secretions, and central and peripheral line tips. Samples were inoculated into Blood agar and MacConkey agar and incubated at 37°C overnight at ambient air. NFGNB were isolated and identified through standard microbiological techniques (colony morphology, Gram staining and motility, and biochemical tests) and by the automated system Vitek 2 Compact (Biomerieux©). Antibiotic susceptibility test was done by both Vitek 2 Compact and Kirby Bauer disc diffusion method following CLSI guidelines 2020.11 Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as control organisms during the study.12 The results were a analyzed, and interpretation was represented graphically in the form of various tables, pie charts, and bar diagrams.
RESULTS
Out of a total of 725 bacterial isolates, 169 NFGNB were isolated with an isolation rate of 23.31%. Of this 169 NFGNB, 84 isolates (49.7%) were Pseudomonas spp., 73 isolates (43.2%) were Acinetobacter spp., seven isolates were B. cepacia (4.14%), and five isolates were S. maltophilia (2.96%) [Figure 1].

- Pie diagram showing the distribution of non-fermenting Gram-negative bacilli into the type of isolated organism.
Males were more commonly affected n = 115 (68%) as compared to females n = 54 (32%) [Figure 2].
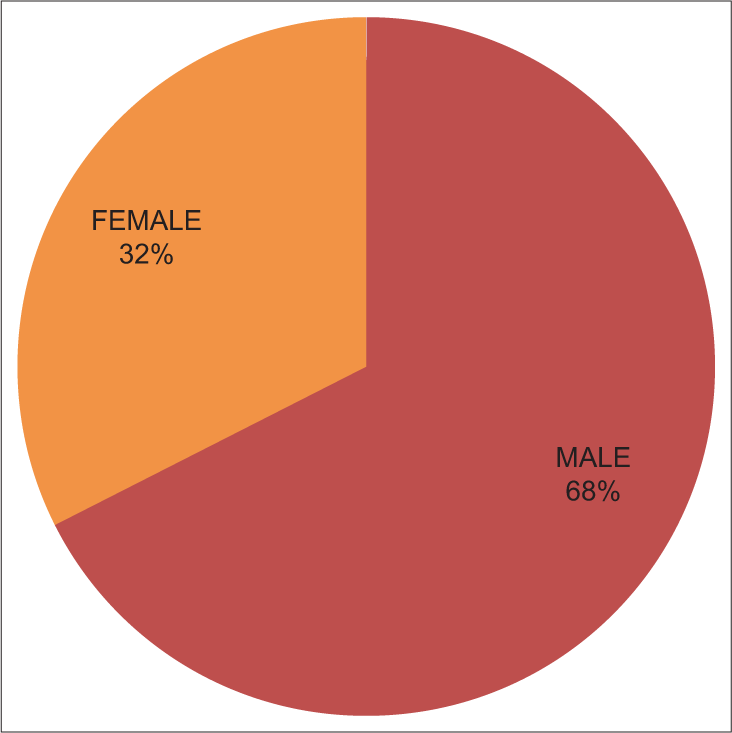
- Pie diagram showing gender distribution of isolated non-fermenting Gram-negative bacilli.
Most of the NFGNB were isolated from pus (40.23%), followed by urine (20.71%), sputum (8.28%), body fluids (7.69%) and central and peripheral line tip (7.1%) [Table 1].
| S. No. | Source of sample | Number | Percentage |
|---|---|---|---|
| 1. | Body fluid | 13 | 7.69 |
| 2. | Blood | 8 | 4.73 |
| 3. | Oral swab | 2 | 1.18 |
| 4. | Urine | 35 | 20.71 |
| 5. | Tissue sample | 3 | 1.77 |
| 6. | Sputum | 14 | 8.28 |
| 7. | Pus | 68 | 40.23 |
| 8. | Et tube aspirate | 9 | 5.32 |
| 9. | Cerebrospinal fluid | 5 | 2.95 |
| 10. | Central line tip | 12 | 7.1 |
Et: Endotracheal
Most isolates of NFGNB were from the department of surgery 60 (35.5%), followed by pediatrics 32 (18.93%), orthopedics 20 (11.83%), hematology 15 (8.87%), chest 12 (7.1%), urology 8 (4.73%) medicine 7 (4.14%), gynecology and obstetrics 6 (3.55%), critical care 4 (2.36%), ENT 3 (1.77%), and endocrine 2 (1.18%) [Figure 3].
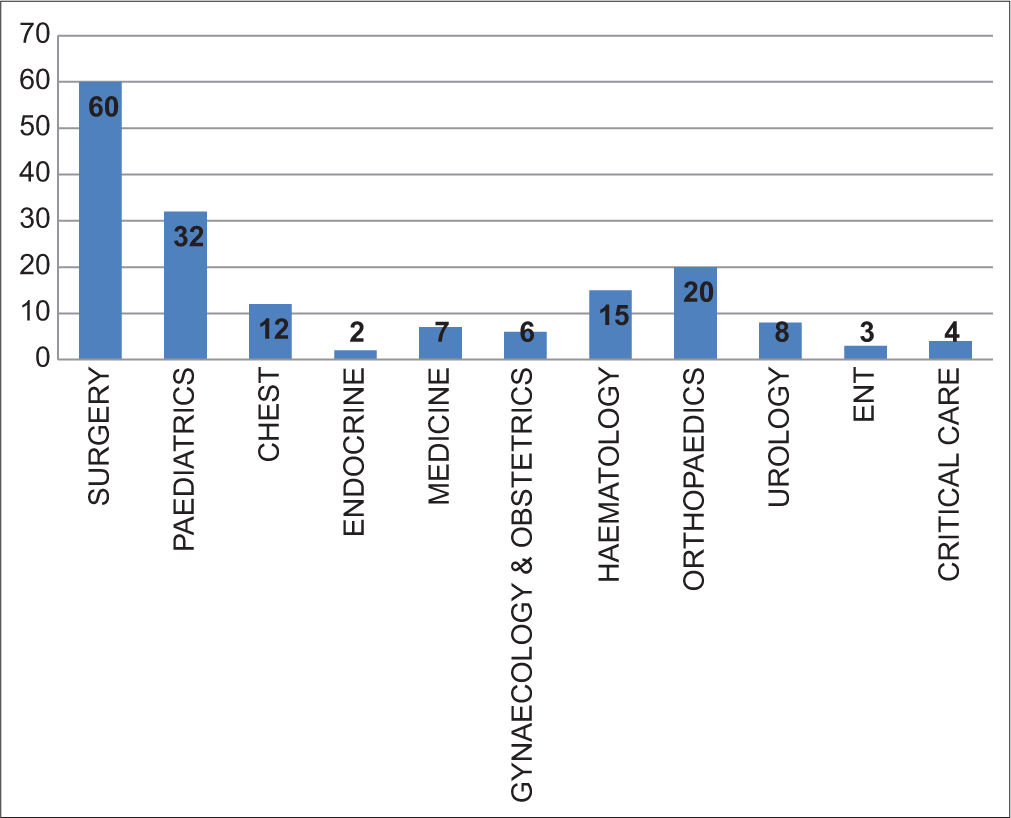
- Bar diagram ward-wise distribution of isolated non-fermenting Gram-negative bacilli.
Antibiotic susceptibility profile of Pseudomonas spp. showed higher resistant pattern against cefepime, levofloxacin, and ticarcillin-clavulanic acid, and organisms were better susceptible to cefoperazone-sulbactam, piperacillintazobactam, and meropenem [Figure 4].
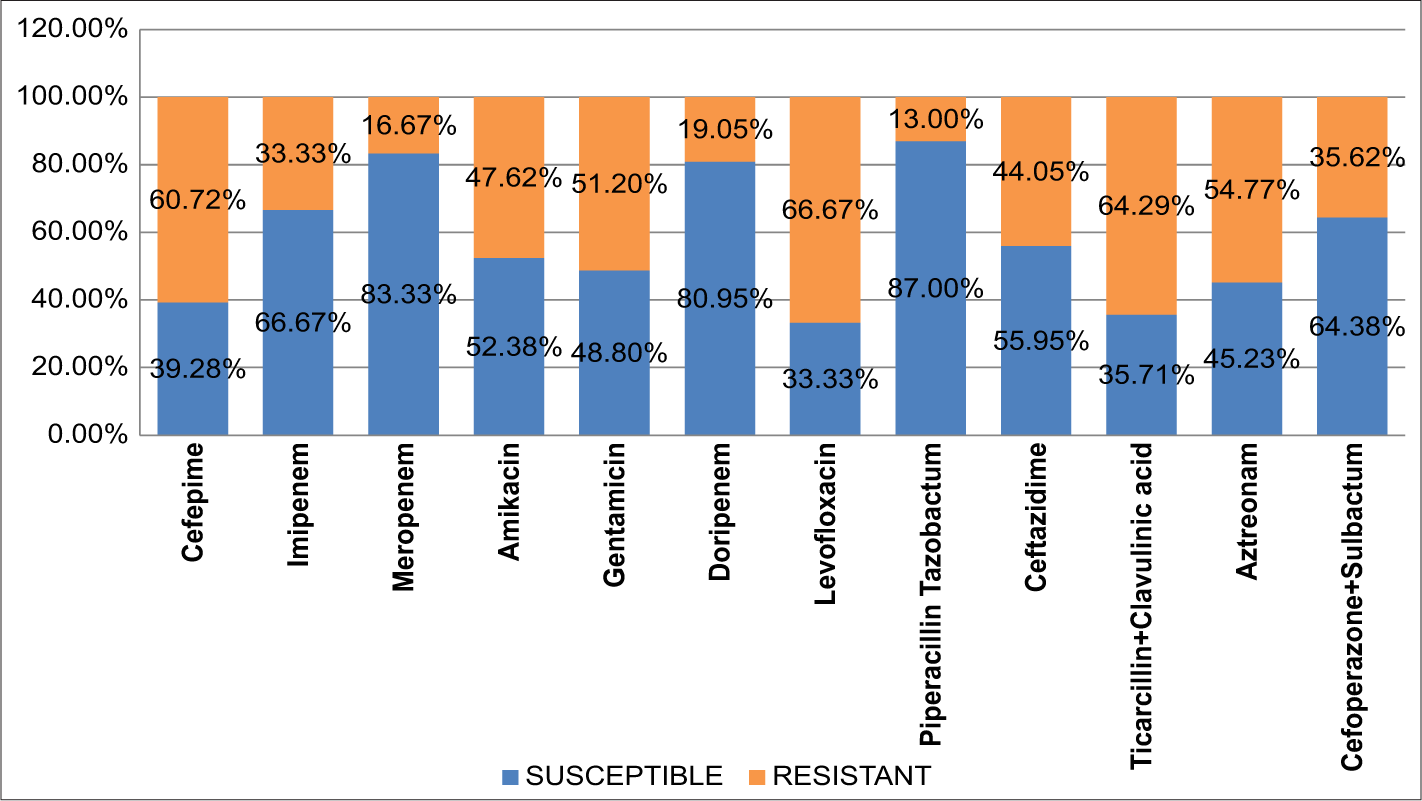
- Bar diagram showing susceptibility pattern in blue color and resistant pattern in green color for Pseudomonas spp.
Among Acinetobacter spp. resistance was more against ceftazidime, levofloxacin, and ticarcillin-clavulanic acid, and better susceptibility was recorded against doripenem, meropenem, and piperacillin-tazobactam [Figure 5].
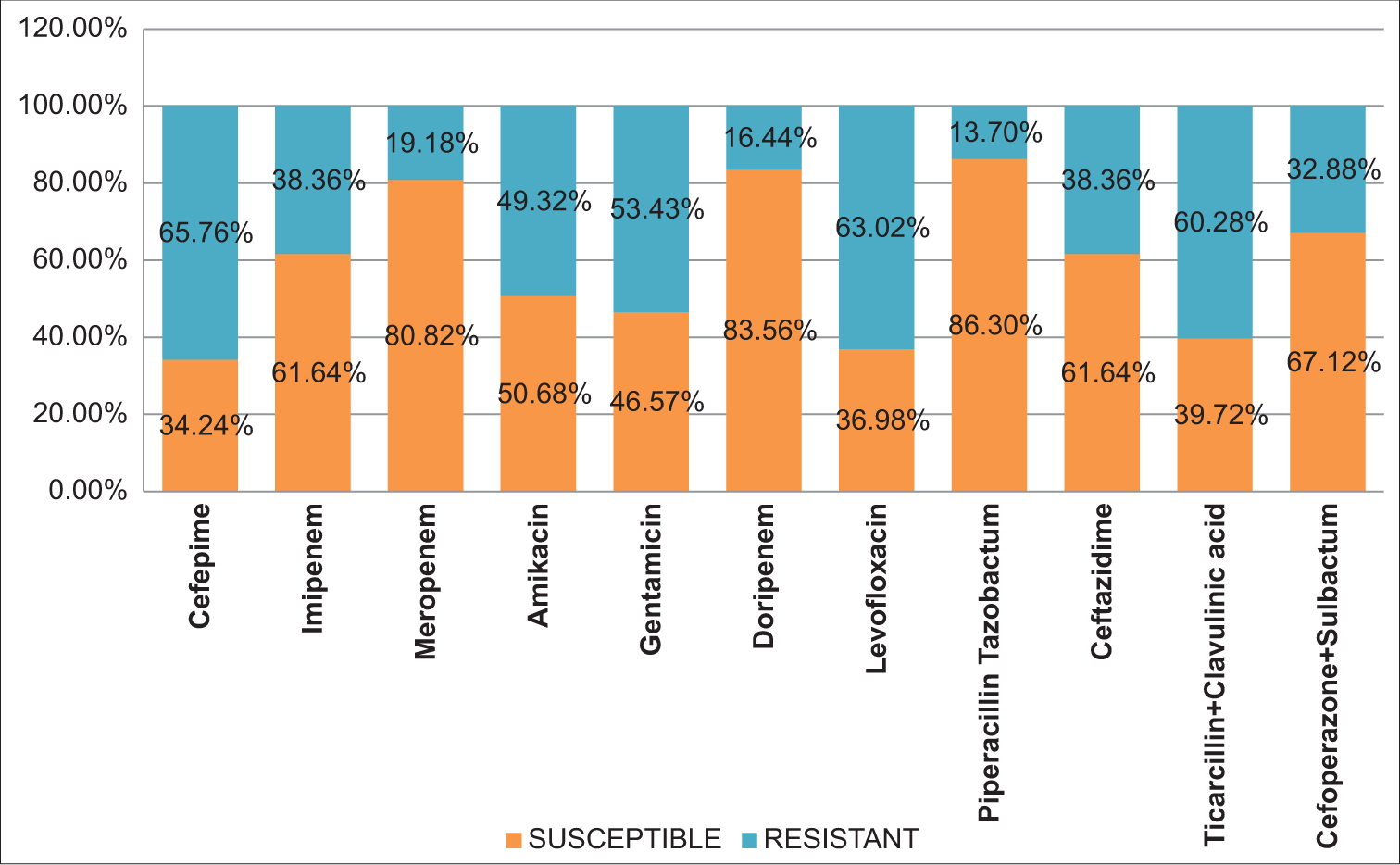
- Bar diagram showing susceptibility pattern in orange color and resistant pattern in blue color for Acinetobacter spp.
B. cepacia showed higher resistance against ticarcillinclavulanic acid (82.16%) and better susceptibility to meropenem 75.29%. S. maltophilia showed better susceptibility against levofloxacin (92.64%), piperacillin-tazobactam (78.31%), minocycline (88.95%), and co-trimoxazole (85.7%).
DISCUSSION
In our study, out of 725 bacterial isolates, 169 isolates were NFGNB, with a proportion of 23.31%. This finding is similar to a study conducted by Vijaya et al.12 (21.8%) and higher than a study conducted by Shastry et al.13 (18.1%) and Kaur et al.14 (16.5%) but lower than by a study done by Deb et al.6 (29.6%) from the same institute. In the present study, the isolation rate of NFGNB out of the total samples was 68% males and 32% females, which is similar to the Jayapriya et al.15 studies, which has reported NFGNB isolates from males 71% and females 29%.
Pseudomonas spp. (49.7%) and Acinetobacter spp. (43.2%) were the common isolates among NFGNB from pus, urine, and body fluids in samples; Deb S et al.6 also showed a higher proportion of Pseudomonas spp. (52.8%) and Acinetobacter spp. (39.3%) isolated from clinical specimens. In our study, the isolation rate of NFGNB from Pus is 42.03% which is lower than Malini et al.16 (62.17%) study. Isolation of NFGNB from urine samples is 21.01% in our study, which is higher than Benanchinmardi et al. (11%) and Malini et al.16,17
In our study, a greater proportion of Pseudomonas spp. (87%) Acinetobacter spp. (86.30%) were susceptible to piperacillin-tazobactam, but in a study by Shastri et al.,13 only 75% of Pseudomonas spp. and 56.5% of Acinetobacter spp. were susceptible. Susceptibility of Pseudomonas spp. and Acinetobacter spp. against meropenem were 83.33% and 80.82%, respectively, which was concordant with the findings of Maniyan et al.,18 they showed that 79.6% of Pseudomonas spp. and 75% of Acinetobacter spp. were susceptible against meropenem.
Pseudomonas spp. and Acinetobacter spp. were least effective against cefepime (60.72% and 65.76% resistant) and levofloxacin (66.67% and 63.02%) that this finding is similar to the finding by Deb et al.,6 they showed 97.1% NFGNB resistant against cefepime and 71% NFGNB resistant to levofloxacin.
Susceptibility profile of other NFGNB, B. cepacia showed higher susceptibility to meropenem, and S. maltophilia was susceptible to levofloxacin, piperacillin-tazobactam, and minocycline in greater proportion, and these findings are similar to findings by Gautam et al.,19 Roy et al.,20 and Namaji et al.21
CONCLUSION
This study highlights the significant presence of NFGNB in clinical samples with a notable proportion of pathogens such as Pseudomonas spp. and Acinetobacter spp. emphasizing the need for heightened awareness and surveillance in health-care settings. The antibiotic susceptibility patterns observed in this study unveil a concerning level of resistance among NFGNB, posing challenges to effective treatment strategies. The emergence of multidrug-resistant strains underscores the urgency of implementing stringent antimicrobial stewardship programs to curb the further spread of resistance. Antibiotic susceptibility patterns in our study are also crucial for clinicians to make informed decisions regarding empirical and targeted antibiotic therapy, ultimately contributing to improved patient outcomes. This study also highlights the importance of ongoing surveillance and continuous monitoring of antibiotic resistance patterns to adapt therapeutic approaches and mitigate the impact of resistant strains on patient outcomes.
Limitation of the study
Molecular mechanism of resistance pattern among NFGNB could not be detected
As this study was conducted during the COVID-19 pandemic, a smaller number of samples were received for processing as compared to the pre-COVID-19 period.
Acknowledgments
The author acknowledges the kind cooperation and support of Mr. Nitish Mondal (Senior Medical Technologist) and Mr. Sayantan Banerjee (MSc Project Scholar) during the study period.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as there were no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Koneman’s Color Atlas and Textbook of Diagnostic Microbiology (7th ed). Philadelphia, PA: Lippincott Williams and Wilkins; 2006. p. :305-91.
- [Google Scholar]
- Infections Due to the Pseudomonas Species and Related Organisms In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, eds. Harrison’s Principles of Internal Medicine (17th ed). USA: McGraw-Hill Medical; 2008. p. :949-56.
- [Google Scholar]
- Prevalence of Non-fermenting Gram-Negative Bacilli and Their in vitro Susceptibility Pattern at a Tertiary Care Teaching Hospital. J Sci Soc. 2014;41:162-6.
- [CrossRef] [Google Scholar]
- Global Challenge of Multidrug-resistant Acinetobacter baumannii. Antimicrobial Agents and Chemotherapy. Am Soc Microbiol. 2007;51:3471-84.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical Site Infection: Clinico-bacteriological Profile and Antibiogram in a Tertiary Care Hospital in Kolkata. Panacea J Med Sci. 2023;13:299-304.
- [CrossRef] [Google Scholar]
- Study of Gram Negative Non Fermenting Bacilli from Surgical Site Infections in a Tertiary Care Hospital in Kolkata. Int J Res Rev. 2019;6:169-74.
- [Google Scholar]
- Bailey and Scott’s Diagnostic Microbiology In: Acinetobacter, Stenotrophomonas and other Organisms (14th ed). St. Louis: Elsevier - Health Science; 2021. p. :362-9. Ch. 20
- [Google Scholar]
- Overview of Nosocomial Infections Caused by Gram-Negative Bacilli. Clin Infect Dis. 2005;41:848-54.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and Outcomes of Health-care-Associated Pneumonia: Results from a Large US Database of Culture-positive Pneumonia. Chest. 2005;128:3854-62.
- [CrossRef] [PubMed] [Google Scholar]
- Relationship between β-Lactamase Production, Outer Membrane Protein and Penicillin-Binding Protein Profiles on the Activity of Carbapenems against Clinical Isolates of Acinetobacter baumannii. J Antimicrob Chemother. 2003;51:565-74.
- [CrossRef] [PubMed] [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing In: CLSI Guideline M100 (30th ed). United States: Clinical and Laboratory Standards Institute; 2020.
- [Google Scholar]
- Prevalence of Non-fermenters in Clinical Specimens. Indian J Med Sci. 2000;54:87-91.
- [Google Scholar]
- A Study of Prevalence and Antimicrobial Susceptibility Pattern of Non Fermenter Gram-Negative Bacilli Isolated From Various Clinical Samples at a Tertiary Care Center, Jaipur. J Clin Med Res. 2019;7:682-9.
- [CrossRef] [Google Scholar]
- Prevalence and Antibiogram of Nonfermenting Gram-Negative Bacilli Isolates Obtained from Various Clinical Samples in a Tertiary Care Hospital, Bathinda, Punjab, India. Int J Res Med Sci. 2018;6:1228.
- [CrossRef] [Google Scholar]
- Nonfermentative Gram Negative Bacilli-Characterisation and Antibiotic Resistant Pattern Study from a Tertiary Care Hospital. Indian J Basic Appl Med Res. 2014;3:227-32.
- [Google Scholar]
- Nonfermenting Gram-Negative Bacilli Infections in a Tertiary Care Hospital in Kolar, Karnataka. J Lab Physicians. 2009;1:62-6.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of Non-fermenting Gram-negative Bacilli and their in vitro Susceptibility Pattern at a Tertiary Care Teaching Hospital. J Sci Soc. 2014;41:162-66.
- [CrossRef] [Google Scholar]
- Characterization and Antimicrobial Susceptibility of Pattern of Non-fermenting Gram Negative Bacilli from Various Clinical Samples in a Tertiary Care Hospital. Indian J Microbiol Res. 2016;3:387-91.
- [Google Scholar]
- Antimicrobial Susceptibility Pattern of Burkholderia cepacia Complex and Stenotrophomonas maltophilia from North India: Trend Over a Decade (2007-2016) Indian J Med Res. 2020;152:656-61.
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial Susceptibility Pattern of Burkholderia cepacia Isolates from Patients with Malignancy. J Glob Infect Dis. 2014;6:90-1.
- [CrossRef] [PubMed] [Google Scholar]
- Antibacterial Susceptibility Pattern of S. Maltophilia Isolates at A Tertiary Care Hospital, India. J Pure Appl Microbiol. 2023;17:2256-62.
- [CrossRef] [Google Scholar]







